| Author | Affiliation |
|---|---|
| Ayan Sen, MD, MSc | Henry Ford Hospital, Department of Emergency Medicine, Detroit, Michigan |
| Joseph Miller, MD | Henry Ford Hospital, Department of Emergency Medicine, Detroit, Michigan |
| Heidi Wilkie, | Henry Ford Hospital, Department of Emergency Medicine, Detroit, Michigan |
| Michele Moyer, BSN | Henry Ford Hospital, Department of Emergency Medicine, Detroit, Michigan |
| Christopher Lewandowski, MD | Henry Ford Hospital, Department of Emergency Medicine, Detroit, Michigan |
| Richard Nowak, MD, MBA | Henry Ford Hospital, Department of Emergency Medicine, Detroit, Michigan |
Introduction
Results
Discussion
Limitations
Conclusion
ABSTRACT
Introduction
Non-invasive, continuous hemodynamic monitoring is entering the clinical arena. The primary objective of this study was to test the feasibility of such monitoring in a pilot sample of Emergency Department (ED) stroke patients. Secondary objectives included analysis of hemodynamic variability and correlation of continuous blood pressure measurements with standard measurements.
Methods
This study was a secondary analysis of 7 stroke patients from a prospectively collected data set of patients that received 2 hours of hemodynamic monitoring in the ED. Stroke patients were included if hemorrhagic or ischemic stroke was confirmed by neuroimaging, and symptom onset was within 24 hours. They were excluded for the presence of a stroke mimic or transient ischemic attack. Monitoring was performed using the Nexfin device (Edwards Lifesciences, Irvine CA).
Results
The mean age of the cohort was 71 ± 17 years, 43% were male, and the mean National Institute of Health Stroke Scale (NIHSS) was 6.9 ± 5.5. Two patients had hemorrhagic stroke. We obtained 42,456 hemodynamic data points, including beat-to-beat blood pressure measurements with variability of 18 mmHg and cardiac indices ranging from 1.8 to 3.6 l/min/m2. The correlation coefficient between continuous blood pressure measurements with the Nexfin device and standard ED readings was 0.83.
Conclusion
This exploratory investigation revealed that continuous, noninvasive monitoring in the ED is feasible in acute stroke. Further research is currently underway to determine how such monitoring may impact outcomes in stroke or replace the need for invasive monitoring.
INTRODUCTION
The management of acute stroke is increasingly relying on frequent measurements of blood pressure, particularly in hemorrhagic stroke and for thrombolytic candidates.1,2 New technologies are making continuous hemodynamic measurements feasible in the emergency department (ED), including measurements of blood pressure and cardiac ouput.3,4 This study explores the ED use of arterial wave-form analysis for capturing continuous hemodynamic measurements in stroke.
Traditionally, blood pressure is measured using standard intermittent oscillometric devices, and monitoring systemic hemodynamics and continuous blood pressure requires invasive technology. Non-invasive devices have been introduced that provide continuous monitoring using algorithms to estimate hemodynamic parameters from blood pressure waveform analysis. Our previous work indicates good correlation of the non-invasive blood pressure measurements using the Nexfin device (Edwards Lifesciences, Irvine, CA) with commonly used cuff measurements using the oscillometric technique.4 Studies have indicated that cardiac output measured by the device closely correlates with pulmonary artery catheter measurements.5,6
This exploratory study assesses the utility of the Nexfin technology to capture blood pressure and systemic hemodynamics in acute stroke patients in the ED. Secondary objectives include assessing variability of beat-to-beat hemodynamic changes, and correlating continuous with standard blood pressure measurements.
Patients and Methods
Study Population
This was a secondary analysis of the subset of stroke patients enrolled in a previously published, observational study of hemodynamic monitoring in critically ill adults.4 The original study prospectively enrolled adults (>18 years) who presented to a resuscitation room at an urban ED over a 4-month period in 2009 as a convenience sample, based on availability of a single trained research assistant. The hospital institutional review board approved the study, and informed consent was obtained prior to enrollment. In the event that patients were unable to give informed consent due to aphasia or mental status changes, consent was obtained from a legally authorized representative.
In this analysis, we included patients from the original study of 40 subjects if their hospital discharge diagnosis was acute stroke, based on confirmed ischemic or hemorrhagic stroke on neuroimaging with symptom onset occurring < 24 hours prior to ED presentation. Patients were excluded from analysis for the following: negative neuroimaging, transient ischemic attack, and presence of stroke mimic determined by treating neurology team.
The Nexfin monitoring is based on the concept of the pulsatile unloading of the finger arterial walls using an inflatable finger cuff with a built-in photoelectric plethysmograph.7–9 While continuously measuring blood pressure, the monitor calculates the cardiac output by the pulse-contour method. The continuous finger pressure is transformed to a brachial artery waveform and the pulsatile systolic area is determined for each heartbeat. Using the arterial impedance, the device calculates cardiac output and stroke volume and determines index values using the patient’s sex, height and weight. A proprietary heart reference system attaches to the patient’s gown at the level of the brachial artery to ensure that the blood pressure values are measured at the same phlebostatic level even if the patient moves his/her hand. Hence, Nexfin readings will automatically correct for any hydrostatic pressure difference and not be falsely elevated or lowered by upward or downward movements of the patient’s hand.
For each patient, a sized finger cuff was placed on the second, third or fourth digit of an asymptomatic hand. Continuous beat-to-beat hemodynamic values from the Nexfin monitor were recorded, including: heart rate, systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), cardiac output, and systemic vascular resistance. The Nexfin device streams data in real-time to a display monitor. For this study, the treating physicians and nurses were blinded to the data. Usual ED cardiac and intermittent blood pressure monitoring continued at hourly intervals based on nursing protocol, and patients were managed as clinically indicated. All patients were contacted after 30 days to determine unexpected, unscheduled ED or healthcare provider visits or death.
Statistical Analysis
We captured and reviewed trends in hemodynamic profiles over a 2-hour period. We analyzed raw data and used moving averages technique to de-noise the beat-to-beat data to remove occasional, artifactual spikes in measurements. Descriptive statistical parameters of the mean and standard deviations of hemodynamic variables were assessed. We computed hemodynamic data over a 30-minute period along with standard deviations to represent variability. We calculated Pearson correlation coefficients to compare Nexfin blood pressure measurements to standard automated measurements and to compare Nexfin derived changes in SBP relative to changes in cardiac index and systemic vascular resistance. All statistical computation was done using SAS 9.2 (Cary, NC).
RESULTS
We initially monitored 7 patients with acute stroke using the Nexfin device, 5 with ischemic and 2 with hemorrhagic stroke. No patients received thrombolytics due to delayed presentation or hemorrhage, and there were no deaths or unscheduled healthcare-related visits in the cohort within 30 days. The mean age of the cohort was 71 ± 17 years and all were African-American; 43% of the cohort was male and the presenting mean National Institute of Health Stroke Scale (NIHSS) was 6.9 ± 5.5 (see Table 1).
Table 1. Demographics of stroke patients.
| Total patients | 7 |
| Male | 3 (43%) |
| Female | 4 (57%) |
| Age (mean ± SD) | 71 ± 17 years |
| NIHSS (mean ± SD) | 6.9 ± 5.5 |
| Total hemodynamic data-points | 42, 456 |
| Hemodynamic data-points (mean ± SD) | 6065 ± 1236 |
| 30-day mortality | 0 % |
NIHSS, National Institute of Health Stroke Scale; SD, standard deviation Table 2Hemodynamic measurements over 30-minute periods.
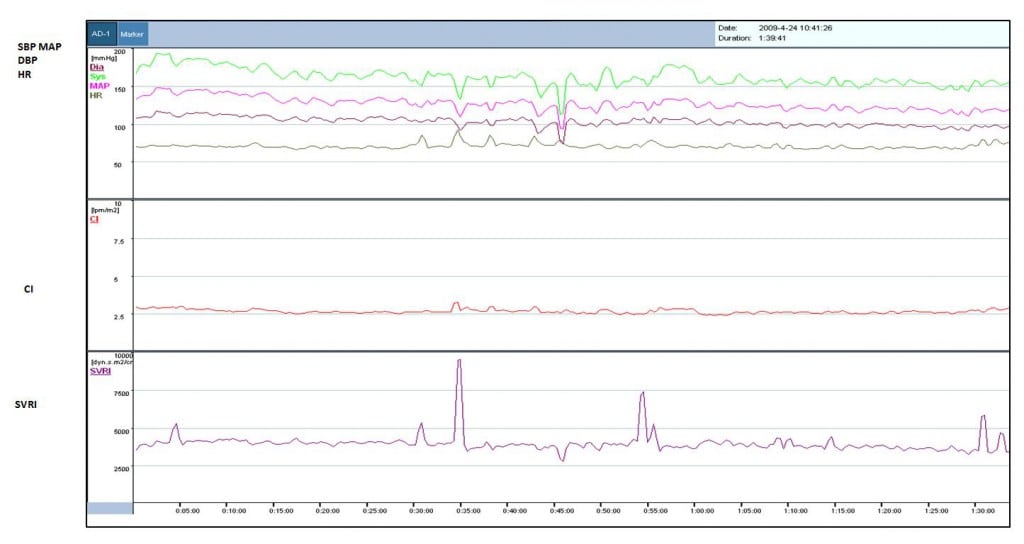
We obtained 42,456 total hemodynamic data points for the 7 patients in the cohort. These data points consist of beat-to-beat measurements of 120 minutes of SBP, DBP, cardiac output, stroke volume, and systemic vascular resistance. A graphical sample of continuous non-invasive monitoring of various hemodynamic parameters of 1 of the 7 patients is shown in Figure 1, including de-noised parameters using the moving averages filter for this patient. After averaging data over 30-minute periods, the cohort’s hemodynamic trends over 2-hours of monitoring are shown in Figure 2. Full hemodynamic statistics for the cohort is listed in Table 2. Variability for the hemodynamic data is represented by the standard deviation. A comparison of all standard automated systolic blood pressure readings and Nexfin values showed a Pearson Correlation Coefficient of 0.83 (p < 0.005).
Figure 1
Single-subject 2-hour trend curve of hemodynamic data using moving averages as measured by Nexfin device.
Figure 2
Hemodynamic trends of cardiac index and systemic vascular resistance index as measured by Nexfin device.
Table 2. Hemodynamic measurements over 30-minute periods.
| Patient (NIHSS) | 1 (4) | 2 (3) | 3 (5) | 4 (7) | 5 (11) | 6 (1) | 7 (17) |
|---|---|---|---|---|---|---|---|
| SBP, mean (SD) | |||||||
| 0–30 minutes | 172 (13) | 133 (23) | 170 (10) | 166 (19) | 177 (22) | 170 (28) | 201 (15) |
| 30–60 | 159 (20) | 144 (22) | 170 (17) | 165 (19) | 168 (20) | 191 (22) | 204 (12) |
| 60–90 | 154 (12) | 142 (25) | 189 (24) | 177 (12) | 173 (19) | 168 (13) | 209 (18) |
| 90–120 | 153 (13) | 153 (25) | 194 (22) | 177 (16) | 178 (16) | 154 (11) | 197 (17) |
| DBP, mean (SD) | |||||||
| 0–30 minutes | 109 (6) | 72 (12) | 72 (4) | 70 (14) | 94 (11) | 81 (19) | 111 (8) |
| 30–60 | 102 (12) | 76 (10) | 73 (7) | 70 (9) | 88 (10) | 83 (11) | 109 (8) |
| 60–90 | 98 (7) | 78 (13) | 81 (10) | 70 (7) | 87 (11) | 72 (6) | 114 (12) |
| 90–120 | 96 (8) | 79 (15) | 83 (9) | 100 (9) | 83 (12) | 66 (5) | 109 (16) |
| MAP, mean (SD) | |||||||
| 0–30 minutes | 136 (9) | 92 (14) | 106 (6) | 107 (14) | 124 (14) | 114 (19) | 146 (11) |
| 30–60 | 125 (14) | 98 (12) | 106 (10) | 105 (11) | 116 (13) | 123 (15) | 145 (10) |
| 60–90 | 120 (9) | 99 (16) | 119 (15) | 109 (11) | 117 (13) | 106 (10) | 141 (12) |
| 90–120 | 118 (10) | 103 (14) | 123 (12) | 72 (9) | 114 (12) | 96 (8) | 128 (19) |
| CI, mean (SD) | |||||||
| 0–30 minutes | 2.7 (0.2) | 2.5 (0.9) | 1.8 (0.1) | 3.1 (0.4) | 3.6 (1.3) | 2.6 (0.8) | 3.0 (0.3) |
| 30–60 | 2.7 (0.7) | 2.6 (1) | 1.8 (0.2) | 3.0 (0.6) | 3.4 (1.3) | 2.8 (0.6) | 3.1 (0.3) |
| 60–90 | 2.6 (0.2) | 2.5 (1.3) | 1.8 (0.4) | 3.2 (0.3) | 3.3 (1.4) | 2.7 (0.4) | 2.9 (0.4) |
| 90–120 | 2.7 (0.4) | 2.4 (1.4) | 1.8 (0.5) | 3.1 (0.5) | 3.6 (1.2) | 2.8 (0.1) | 2.6 (0.7) |
| SVRI, mean (SD) | |||||||
| 0–30 minutes | 4070 (1342) | 3865 (6772) | 4738 (790) | 3082 (4899) | 3312 (2474) | 4420 (5692) | 3913 (1158) |
| 30–60 | 4088 (4895) | 4063 (5039) | 4910 (1220) | 3475 (8893) | 3207 (1777) | 3863 (4433) | 3864 (1662) |
| 60–90 | 3789 (1194) | 4887 (7603) | 5428 (2807) | 2782 (1497) | 3507 (2371) | 3225 (2941) | 3671 (1893) |
| 90–120 | 4474 (2085) | 4945 (9521) | 5572 (4079) | 2980 (2287) | 3682 (1746) | 2715 (3150) | 3447 (1921) |
| HR, mean (SD) | |||||||
| 0–30 minutes | 70 (3) | 98 (20) | 54 (4) | 70 (7) | 110 (27) | 66 (16) | 77 (7) |
| 30–60 | 73 (12) | 105 (20) | 53 (7) | 70 (10) | 104 (27) | 66 (11) | 74 (5) |
| 60–90 | 69 (5) | 106 (27) | 56 (10) | 69 (4) | 92 (23) | 61 (7) | 72 (12) |
| 90–120 | 72 (12) | 96 (34) | 60 (17) | 70 (7) | 97 (19) | 60 (3) | 68 (11) |
SBP, systolic blood pressure; SD, standard deviation; MAP, mean arterial pressure; DBP, diastolic blood pressure; HR, heart rate; CI, cardiac index; SVRI, systemic vascular resistance index
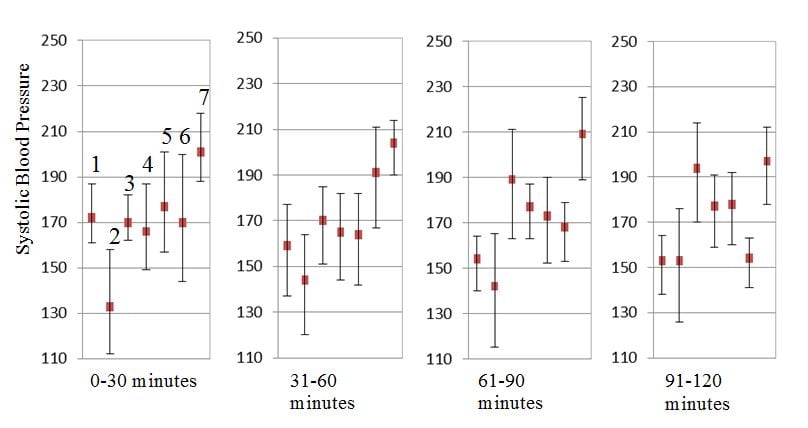
The average variability of SBP, DBP and MAP for the cohort was 18.1 mmHg, 9.9 mmHg and 12.2 mmHg respectively. The variability in SBP for each patient over 30-minute increments is represented by the standard deviation lines shown in Figure 3. The cohort’s range of cardiac indices was 1.8 to 3.6 l/min/m2, and the range for the systemic vascular resistance indices was 2715 to 5572 dynes·sec/cm5/m2. Changes in blood pressure correlated poorly with changes in systemic vascular resistance and cardiac output. The correlation coefficients were -0.21 (-0.46 to 0.07, 95% CI) and 0.24 (-0.04 to 0.48, 95% CI) respectively.
Figure 3
Variability in systolic blood pressure measured by Nexfin device. Vertical lines around each mean value represent standard deviation and show variability in systolic blood pressure for each study patient.
DISCUSSION
This analysis reveals the feasibility of non-invasive, continuous blood pressure and hemodynamic monitoring in acute stroke. Due to the invasive nature of prior methods for determining hemodynamic measurements, there is little data on hemodynamic changes in stroke and their relationship to outcomes. Nevertheless, identification of high-risk hemodynamic profiles in stroke could lead to better risk stratification and potential interventions to improve clinical outcomes. Our group is currently investigating the relationship between hemodynamic profiles in stroke, anatomic location and alterations in cerebral blood flow.10
Continuous monitoring may be particularly of interest in hemorrhagic stroke, as recent literature indicates a role for more aggressive blood pressure lowering.1 The continuous measurements by the Nexfin are displayed on a portable screen to the treating clinician equivalent to arterial line monitoring. Although we used minute-averaging techniques to smooth the data trends for data analysis, a clinician can readily use the real-time measurements to make management decisions in lieu of arterial-line or oscillometric measurements. Such monitoring may also be of interest in managing patients that are thrombolytic candidates. These patients have a short time to reach goal blood pressure (< 185/110 mmHg) prior to thrombolytic administration, and if they receive thrombolytics, guidelines recommend vigilant maintenance of SBP < 180 mmHg.2
Finally, the ability to detect variability in blood pressure may have prognostic significance in stroke. Fluctuations early in the course of ischemic stroke are associated with poor 90-day survival.11 A study done within 72-hours of stroke onset, which obtained 10-minute continuous recordings, found that high arterial pressure variability was associated with a poor outcome, defined as death or dependency.12 Our data show similar variability of blood pressure in acute stroke in the initial hours of presentation, although this analysis is underpowered to assess outcomes. The wide standard deviation values for SBP, DBP and MAP point to this significant intra- and inter-individual variability (Table 2 and Figure 3).
LIMITATIONS
Limitations of our study include the small sample size, exploratory analysis, mixed stroke etiologies and convenience sampling methods. The sample size is too small to make any meaningful patient-centered conclusions. Additional methodological limitations were the inclusion of stroke patients with varying times of symptom onset within a 24-hour period and the fact that stroke anatomic location was not recorded. Finally, the Nexfin derived hemodynamic measurements have been validated against thermodilution techniques but are not the gold standard measurements.
CONCLUSION
This exploratory investigation reveals that continuous, non-invasive monitoring of blood pressure and cardiac hemodynamic variables is feasible in acute stroke. As non-invasive monitoring technology advances, there may be a role for improved monitoring, prognostication and interventions based on robust hemodynamic data in a previously data-poor area of critical illness.
Footnotes
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Address for Correspondence: Ayan Sen, MD, M.Sc. is currently with the Department of Critical Care Medicine, Mayo Clinic Arizona. This study was conducted at the Department of Emergency Medicine, Henry Ford Hospital, 2799 W. Grand Blvd., Detroit, MI 48202. Email: ayan2024@gmail.com. 7 / 2014; 15:345 – 350
Submission history: Revision received February 8, 2013; Submitted March 31, 2014; Accepted April 23, 2014
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources and financial or management relationships that could be perceived as potential sources of bias. The device used in this project was provided by BMEYE, Amsterdam, along with an unrestricted education grant. The device manufacturer had no role in study design, data analysis, preparation or review of the manuscript.
REFERENCES
1 Anderson CS, Heeley E, Huang Y Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. 2013; 368:2355-65
2 Jauch EC, Saver JL, Adams HP Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013; 44:870-947
3 Ramirez MF, Tibayan RT, Marinas CE Prognostic value of hemodynamic findings from impedance cardiography in hypertensive stroke. Am J Hypertens. 2005; 18:65S-72S
4 Nowak RM, Sen A, Garcia AJ Noninvasive continuous or intermittent blood pressure and heart rate patient monitoring in the ED. Am J Emerg Med. 2011; 29:782-9
5 Bogert LW, Wesseling KH, Schraa O Pulse contour cardiac output derived from non-invasive arterial pressure in cardiovascular disease. Anaesthesia. 2010; 65:1119-25
6 Broch O, Renner J, Gruenewald M A comparison of the Nexfin and transcardiopulmonary thermodilution to estimate cardiac output during coronary artery surgery. Anaesthesia. 2012; 67:377-83
7 Akkermans J, Diepeveen M, Ganzevoort W Continuous non-invasive blood pressure monitoring, a validation study of Nexfin in a pregnant population. Hypertens Pregnancy. 2009; 28:230-42
8 Eeftinck Schattenkerk DW, van Lieshout JJ, van den Meiracker AH Nexfin noninvasive continuous blood pressure validated against Riva-Rocci/Korotkoff. Am J Hypertens. 2009; 22:378-83
9 Hofhuizen CM, Lemson J, Hemelaar AE Continuous non-invasive finger arterial pressure monitoring reflects intra-arterial pressure changes in children undergoing cardiac surgery. Br J Anaesth. 2010; 105:493-500
10 Miller JB Henry Ford Hospital Hemodynamic Effects on Cerebral Autoregulation in Acute Stroke. ClinicalTrialsgov [Internet]. 2000;
11 Stead LG, Gilmore RM, Vedula KC Impact of acute blood pressure variability on ischemic stroke outcome. Neurology. 2006; 66:1878-81
12 Hickey JV, Salmeron ET, Lai JM Twenty-four-Hour blood pressure variability after acute ischemic stroke. Crit Care Nurs Q. 2002; 25:1-12