| Author | Affiliation |
|---|---|
| Kevin Shah, BS | Veterans Administration San Diego Healthcare System, Cardiology, La Jolla, CA |
| Garrett J. Terracciano, BA | University of California, San Diego School of Medicine, La Jolla, CA |
| Kevin Jiang, BS | Veterans Administration San Diego Healthcare System, Cardiology, La Jolla, CA University of California, San Diego School of Medicine, La Jolla, CA |
| Alan S. Maisel, MD | Veterans Administration San Diego Healthcare System, Cardiology, La Jolla, CA |
| Robert L. Fitzgerald, PhD | University of California, San Diego School of Medicine, La Jolla, CA VA San Diego Healthcare System, Pathology, La Jolla, CA |
ABSTRACT
Introduction:
Heart failure is one of the leading causes of death in the U.S. The incorporation of B-type natriuretic peptide (BNP) measurements when triaging patients presenting with shortness of breath has improved the diagnostic and prognostic ability of physicians. Currently, there are no point-of-care systems for quantifying BNP that can be used without sacrificing accuracy. We compared the analytical performance of the Abbott i-STAT analyzer, a handheld point-of-care system for measuring BNP, with the lab-based system, the Abbott ARCHITECT.
Methods:
One-hundred fifty samples were collected from three clinical settings: 41 from the Emergency Department, 58 from the inpatient wards, and 51 from heart failure outpatient clinics. Linear regression and bias difference analyses were run to evaluate the accuracy of the i-STAT. Correlation between the i-STAT and Architect BNP values were made with values of BNP.
Results:
The correlation coefficient was r=0.977 (N=150, p<.0001). The average bias was significant (-36) and there were concentration-dependent differences at higher BNP values. Precision of the i-STAT was poor compared to the lab-based platform.
Conclusion:
Although the precision of the i-STAT was poor, there was good clinical agreement between the i-STAT and the lab-based platform.
INTRODUCTION
Heart failure (HF) is one of the leading causes of death in the U.S. About five million Americans have this disease, and approximately 550,000 new cases are identified each year.1 The estimated direct and indirect cost of HF in the U.S. for 2006 was $29.6 billion.1 With improving diagnosis and management of acute myocardial infarction and HF, it is likely this cost will continue to increase over time. The number of HF-related hospital admissions has been steadily rising in developed countries. The economic burdens of HF are caused by the high number of hospital admissions for initial treatment and high costs of long term care for these patients.2 While the most common disease group in patients over 65 is HF,2 it remains difficult to diagnose due to a lack of sensitive and specific presenting symptoms.3 Furthermore, a misdiagnosis in the emergency department (ED) could place a dyspneic patient at increased risk for both morbidity and mortality.4 The “gold standard” for diagnosis is echocardiography, which is not generally available in the emergency setting. Due to the alarming costs of HF, there is an urgent need to detect patients at risk of developing HF and establishing timely therapy to prevent irreversible changes that can lead to chronic HF.
Incorporation of B-type natriuretic peptide (BNP) measurements when triaging patients presenting with shortness of breath has improved the diagnostic and prognostic ability of treating physicians. In the “Breathing Not Properly Multinational Study,” in 1,586 ED patients presenting with acute shortness of breath, BNP levels measured on arrival had higher diagnostic accuracy than did the ED physician in diagnosing HF, with an area under the receiver-operating characteristic curve (AUC) of 0.90.5 A BNP cut-point of 100 pg/mL was 90% sensitive and 76% specific for diagnosing HF as the cause of dyspnea.
Current turnaround times for BNP values, including time to draw sample, transport to central lab, analyze and report values, using lab-based automated analyzers on ED patients is typically around one hour.
Shortening this turnaround time in the emergent setting could potentially help physicians make a more rapid “rule-in” or “rule-out” diagnosis of HF. Mueller et al.6 and Troughton et al.7demonstrated that rapid evaluation of BNP in HF patients shortened the time to treatment initiation, decreased the time to discharge, decreased the total medical costs for that patient, reduced total cardiovascular events, and delayed time to first event.
Attempts at providing a more rapid, point-of-care (POC) BNP test have suffered from analytical, regulatory, and management issues. Our objective in this study was to compare the analytical performance of the POC i-STAT® system for measuring BNP levels with a standard, lab-based ARCHITECT® instrument (Abbott Laboratories, Abbott Park, IL).
METHODS
Patients for this study were enrolled from the ED, inpatient setting, and heart failure clinics at the San Diego Veterans Affairs Healthcare System between January 2007 and January 2008. There were 114 patients, with 41 samples collected from the ED setting, 58 samples from the inpatient setting, and 51 samples from the clinic/outpatient setting. Thirty-six patients from the ED were later admitted and were sampled again as inpatients. Distribution of patients included 110 males (mean age 68, range 38–90 yrs) and four females (mean age 59, range 46–83 yrs.). Inclusion criteria were presentation with heart failure (HF) symptoms in the ED, hospitalization for HF, or visitation in a heart failure clinic. Patients on dialysis, patients with trauma-related shortness of breath and patients unwilling to sign a consent form were not enrolled in the study. The study was approved by review through the Institutional Review Board at the University of California, San Diego.
The i-STAT BNP test is a handheld in vitro diagnostic test for the quantitative measurement of BNP. The i-STAT BNP cartridge uses a two-step sandwich immunoassay using monoclonal antibodies specific for BNP. The i-STAT BNP test uses the same antibodies as the ARCHITECT BNP assay. The capture antibody recognizes amino acids 5 to 13 and the detection antibody that recognizes amino acids 26 to 32 of the BNP molecule.10 The i-STAT analyzer and BNP cartridges and controls were provided by Abbott, as was the ARCHITECT. Statistical analysis was conducted using SigmaPlot (Systat, San Jose, CA).
Lavender-top, ethylenediaminetetraacetic acid (EDTA) whole blood specimens were obtained in plastic tubes; plasma was isolated and stored at −80 degrees Celsius until analysis. Samples were thawed and analyzed weekly in batches on the ARCHITECT and i-STAT. The ARCHITECT and i-STAT measurements were performed at the same time. All personnel running the devices were laboratory staff, trained on the operation of both devices. Linearity and precision of each device or assay was performed using materials provided by the manufacturer.
Linear regression analyses were done and bias differences were analyzed according to Bland and Altman.11 Within-run and run-to-run precision data was obtained on the i-STAT analyzer at both a high and low level. High and low controls were run on the i-STAT analyzer 15 times in one day for within-run calculations. High- and low-level precision controls were run on the i-STAT analyzer twice a day for 15 days for run-to-run calculations. High- and low-level precision controls were run on the ARCHITECT 15 times in one day for within-run calculations. High- and low-level precision controls were run on the ARCHITECT in duplicate twice a day for five days for run-to-run calculations. An F test was used to compare the variance of the i-STAT and the ARCHITECT. A p-value < 0.05 was considered significant.
RESULTS
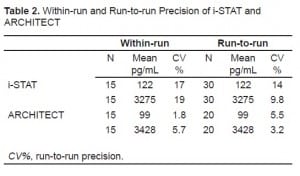
Sample population characteristics and median lab values are shown in Table 1. Results of precision testing comparing the i-STAT and ARCHITECT are shown in Table 2. For the i-STAT, run-to-run precision (%CV) for the low level (mean=122 pg/mL) was 14% and for the high level (mean=3274 pg/mL) 9.8%. Within-run precision on the i-STAT analyzer for the low level was 17% and for the high level 19%. For the ARCHITECT instrument, run-to-run precision (%CV) for the low level (mean=99 pg/mL) was 5.5% and for the high level (mean=3428 pg/mL) 3.2%. Within-run precision using the ARCHITECT instrument for the low level was 1.8% and 5.7% for the high level.

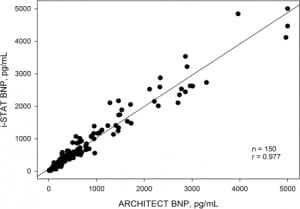
To determine the accuracy of the BNP i-STAT analyzer, we compared results with those obtained on the ARCHITECT. The range of BNP values obtained on patients for the i-STAT and ARCHITECT was 5 – 5000 pg/mL. Correlations between plasma measurements made with i-STAT and those made with ARCHITECT are shown in Figure 1. Linear regression between the i-STAT and the reference system showed r=0.977 (p<.0001).
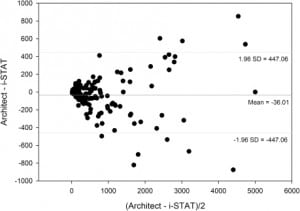
In Figure 2, a difference bias plot for the total sample population was constructed according to Bland and Altman.9 The average bias was −36.01 with significant concentration-dependent differences at higher BNP values.
DISCUSSION
In this study we evaluated the performance of a handheld POC BNP analyzer against a standard lab-based model. In terms of correlation with the laboratory analyzer, the i-STAT analyzer performed remarkably well with a cumulative correlation of 0.977 for the entire cohort. However, the precision of the i-STAT was poor as compared with the ARCHITECT system.
POC testing for BNP, similar to use of troponins in ACS, is a logical progression in the treatment and risk stratification of patients with HF; however, the i-STAT, with its unique strengths (decreased therapeutic turnaround time, rapid data availability, accuracy) and weaknesses (precision, handling error, cost), proves to be most useful in the emergent setting as a bridge to the inpatient stay and subsequent lab-based analyses. The main weakness of the i-STAT BNP, like other POC systems, is its lack of precision. From the emergency physician’s (EP) perspective, knowledge of the BNP upon presentation is important in guiding treatment plan. The positive predictive value of BNP at 100 pg/mL is 75% and 86% at 400 pg/mL.5 In addition, a presenting BNP value of below 200 pg/mL is an excellent predictor of 90-day prognosis.8 While a BNP value of over 480 pg/mL was a strong predictor of 6-month CHF death, hospital readmission, or repeat ED visits.9 Therefore, knowledge of a specific BNP value (not just a “rule-in” vs. “rule-out” value) is useful to the EP in directing treatment of a patient presenting with CHF exacerbation. The average bias of −36.01 pg/mL and the relatively poor precision of the i-STAT analyzer has to be taken into account when considering implementation of the device, because the difference in precision does make a clinical difference in managing a patient with CHF exacerbation.
The strength of the i-STAT BNP complements the current evolution of the treatment and management of HF as a disease. Once thought to be simply a hemodynamic instability where a patient was either “in” HF or “out of” HF, there is now a greater appreciation for the progressive nature of the disease. Recognizing that remodeling of the heart is constant and gradated means treatment no longer focuses solely on the hemodynamics (diuretics and digitalis) but also includes attention to the heart as a dynamic neuroendocrine organ (ACE inhibitors).12 The need for a rapid POC system to evaluate BNP in HF patients is two-fold: rapidity and integration into HF management.
While the ARCHITECT instrument takes less than 16 minutes to analyze BNP values, turnaround times of results generally less than one hour due to specimen-processing and transportation issues. The i-STAT analyzer takes only 10 minutes and can be readily available in the ED. Early assessment of the degree of hemodynamic compromise (measured indirectly with BNP) allows physicians to make the appropriate medical interventions necessary to minimize damage to the heart. A rapidly obtained BNP value could prove useful in the ED setting or in the clinic setting, for ruling in/out HF. Furthermore, previous studies have shown that shortened times to BNP evaluation in the emergent setting resulted in quicker treatment initiation and shorter hospital stays.3
Cost analysis is another factor to be considered when contrasting the i-STAT analyzer with the ARCHITECT instrument. Studies have shown that the cost of using POC testing over central laboratory can increase costs 1.1 to 4.6 times.13 The ability to gain a rapid BNP value in the emergent setting would most likely come at a higher cost than continuous use of a central laboratory. However, the i-STAT analyzer, providing a more rapid result, would lead to shorter hospital stays which would reduce costs for the institution.3 Fiscal considerations should be urged before erroneously implementing this platform into a hospital setting.
LIMITATIONS
The study population was drawn from the Veterans Affairs Medical Center and was 96% male. Thus, these results need to be confirmed in a non-Veterans Affairs population and with women. In addition, the analyses were not performed in real time. Samples were frozen and batch-run, so the study can be considered “in-vitro.” However, the strength of the i-STAT remains valuable to the EP in the event of an emergent presentation.
CONCLUSION
Previous POC systems correlate poorly with the lab-based instruments, making it difficult for the physician to interpret and track changes in the BNP levels from ED presentation throughout the hospital stay.14 In contrast, the i-STAT BNP test utilizes the same antibody as the ARCHITECT BNP assay and, consequently, demonstrates reasonable agreement between the two methods. This provides essential continuity in tracking BNP changes throughout the hospital stay and could allow for accurate charting of a patient’s response to therapy. The emerging technology of a rapid POC BNP assay allows physicians to rapidly triage patients and may allow physicians to initiate treatment more rapidly. This could potentially reduce medical costs and total cardiovascular events. Although the i-STAT lacks precision, it could serve as a practical tool in the clinic setting and emergent setting for acquiring a rapid initial BNP value for managing patients.
Footnotes
The authors are indebted to the efforts of the hospital staff at the Veterans Affairs Medical Center in San Diego, CA. Funding and support was provided by Abbott Laboratories, Inc.
Supervising Section Editor: Jeffrey Sankoff, MD
Submission history: Submitted February 24, 2009; Revision Received August 5, 2009; Accepted August 22, 2009
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Address for Correspondence: Kevin S. Shah, VA San Diego Healthcare System, 3350 La Jolla Village Drive, Cardiology Section, mc 111A, San Diego, CA 92161
Email: Kevin.shah@uci.edu
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The following financial disclosures are related to Abbott Laboratories: Alan S. Maisel, MD –received research support.
REFERENCES
1. American Heart Association. Heart Disease and Stroke Statistics: 2005 Update. Dallas, TX: American Heart Association; 2005.
2. McMurray JJV, Stewart S. The burden of heart failure. Eur Heart J Suppl. 2002;4:50–58.
3. Stevenson LW, Perloff JK. The limited availability of physical signs for estimating hemodynamics in chronic heart failure. JAMA. 1989;261:884–8. [PubMed]
4. Wuerz RC, Meador SA. Effects of pre hospital medications on mortality and length of stay in CHF.Ann of Emerg Med. 1992;21(6):669–74. [PubMed]
5. Maisel AS, McCord J, Nowak RM, et al. Breathing Not Properly Multinational Study Investigators. Bedside B-Type natriuretic peptide in the emergency diagnosis of heart failure with reduced or preserved ejection fraction. Results from the Breathing Not Properly Multinational Study. J Am Coll Cardiol. 2003;41(11):2018–21. [PubMed]
6. Mueller C, Scholer A, Laule-Kilian K, et al. Use of B-type natriuretic peptide in the evaluation and management of acute dyspnea. N Engl J Med. 2004;350(7):647–54. [PubMed]
7. Troughton RW, Frampton CM, Yandle TG, et al. Treatment of heart failure guided by plasma aminoterminal brain natriuretic peptide (N-BNP) concentrations. Lancet. 2000;355:1126–30.[PubMed]
8. Maisel AS, Hollander JE, Guss D, et al. Primary results of the Rapid Emergency Department Heart Failure Outpatient Trial (REDHOT): A multicenter study of B-type natriuretic peptide levels, emergency department decision making, and outcomes in patients presenting with shortness of breath. J Am Coll Cardiol. 2004;44(6):1328–33. [PubMed]
9. Harrison, Morrison LK, Krishnaswamy P, et al. B-type natriuretic peptide predicts future cardiac events in patients presenting to the emergency department with dyspnea. Ann Emerg Med.2002;39:131–8. [PubMed]
10. Rawlins M, Owen WE, Roberts WL. Performance Characteristics of Four Automated Natriuretic Peptide Assays. Am J Clin Pathol. 2005;123:444.
11. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–10. [PubMed]
12. Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation.2005;112(12):e154–235. [PubMed]
13. Nosanchuk JS, Keefner R. Cost analysis of point-of-care laboratory testing in a community hospital. Am J Clin Pathol. 1995;103(2):240–3. [PubMed]
14. Kost GJ, Ehrmeyer SS, Chernow B, et al. The Laboratory-Clinical Interface: Point-of-Care Testing.Chest. 1999;115(4):917–8. [PubMed]


