| Author | Affiliation |
|---|---|
| Si-Kyung Jung, MD, PhD | Catholic University of Korea, Department of Emergency Medicine, Seoul, South Korea |
| Yeon Hee Jeong, MD | Catholic University of Korea, Department of Emergency Medicine, Seoul, South Korea |
| Woon Jeong Lee, MD | Catholic University of Korea, Department of Emergency Medicine, Seoul, South Korea |
| Carol Lee, MD | Harbor-UCLA Medical Center, Department of Emergency Medicine, Torrance, California |
| Amy H. Kaji, MD, PhD | Harbor-UCLA Medical Center, Department of Emergency Medicine, Torrance, California |
| Roger J. Lewis, MD, PhD | Harbor-UCLA Medical Center, Department of Emergency Medicine, Torrance, California |
ABSTRACT
Introduction:
In the last several decades, South Korea has rapidly adopted Western customs and practices. Yet, cultural differences between South Korea and the United States exist. The purpose of this study was to identify and characterize potential cultural differences in the Korean and US institutional review board (IRB) approach to certain topics.
Methods:
A qualitative analysis of a 9-item survey, describing 4 research study case scenarios, sent to IRB members from the United States and South Korea. The case scenarios involved the following issues: (1) the need for consent for retrospective chart review when research subjects receive their care after the study is conceived; (2) child assent; (3) individual versus population benefit; and (4) exception from informed consent in emergency resuscitation research. The free-text responses were analyzed and abstracted for recurrent themes.
Results:
Twenty-three of the 45 survey recipients completed the survey, for an overall response rate of 51%. The themes that emerged were as follows: (1) the importance of parental authority among Korean participants versus the importance of child autonomy and child assent among US participants; (2) the recognition of the rights of a proxy or surrogate who can represent an individual’s values by all participants; and (3) the importance of the community, expressed by the Korean respondents, versus individualism, expressed by US respondents.
Conclusion:
Whereas US participants appear to emphasize the importance of the individual and the autonomy of a child, the Korean respondents stressed the importance of parental authority and benefiting the community, above and beyond that of the individual person. However, there was substantial overlap in the themes expressed by respondents from both countries.
INTRODUCTION
Defining the code of ethics in medical research, the Nuremberg code was developed after the Second World War and emphasizes the principles of informed consent, absence of coercion, and beneficence.1 In 1974, the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research created the institutional review board (IRB) to further help protect the rights and welfare of the human research subjects.2 In response to the Tuskegee Syphilis Study (1932–1972), the Commission published the “Ethical Principles and Guidelines for the Protection of Human Subjects of Research,” or the Belmont Report,3 which describes a number of basic ethical principles: respect for persons in protecting an individual’s autonomy; beneficence in minimizing harm to research subjects while maximizing research benefit; justice in ensuring that research procedures are reasonable, fair, and equitable; and nonmaleficence in doing no harm. To this day, the Belmont Report provides the moral framework upon which an IRB ensures that human research projects meet ethical regulations.
As Korea begins conducting medical research, clinical researchers are faced with developing the technical infrastructure to conduct sound research but also the moral and ethical infrastructure to provide human subjects’ protection. Until mid 1980 in Korea, there were no guidelines for obtaining informed consent, reporting adverse reactions, or compensating research subjects. Shortly thereafter, recognizing the need for regulation in clinical research and drug development, the Korean government formed the Korean Good Clinical Practice Committee (KGCP).4 In 1995, the KGCP mandated that all IRBs review and monitor clinical trials, and in 2001, the KGCP encouraged the IRBs to adhere to international ethics guidelines. Yet, because there continued to be great variability among IRB practices, the Korean Association of Institutional Review Boards (KAIRB) was formed. Comprised of IRB members from major hospitals, biomedical researchers, medical directors of pharmaceutical companies, and officers from health authorities, KAIRB aimed to help Korean IRBs conform to international medical ethics standards.5
Over the last several decades, Korea has rapidly adopted Western customs and practices. However, numerous social and cultural differences between the United States and Korea remain. Given the cultural differences between Korea and the United States, differences in the IRB approach to research between the countries may be expected. Yet, while the interpretation of a given research project may depend upon cultural norms and mores, every research project should nonetheless be ethically sound. The purpose of this study was thus to explore similarities and differences in the evaluations of human subjects’ studies between the IRBs in the United States and South Korea.
METHODS
Study Design
This is a qualitative analysis of a 9-item survey, describing 4 research study case scenarios, which were sent to IRB members from the United States and South Korea, via either air mail or US parcel post. The survey instrument was developed to help characterize differences between the 2 countries’ approaches to human subjects’ protection and consent issues. A Korean bilingual medical professional translated the English version of the survey instrument into Korean, which was then reviewed by another independent Korean medical professional to ensure adequacy of translation. The free-text survey responses from the Korean participants were translated by a single Korean bilingual medical professional.
The survey items, based upon 4 study case scenarios, were aimed at identifying and characterizing potential cultural differences in the Korean and US IRB approach to certain topics: (1) the need for consent for retrospective chart review when research subjects receive their care after the study is conceived; (2) child assent; (3) individual versus population benefit; and (4) exception from informed consent in emergency resuscitation research. Since Korean culture developed out of Confucian traditions, whereas the US was founded upon European, Judeo-Christian philosophies, we expected to identify some differences.
The study protocol was approved by the Human Subjects Committee of the Harbor-University of California, Los Angeles (UCLA) Biomedical Research Institute.
Case Study Scenarios
The 4 scenarios depicted in the survey instrument are described below.
Scenario 1: Study subjects included patients presenting to the emergency department with tissue hypoperfusion and systemic inflammatory response who are to be treated by using the Rivers protocol (ie, early goal-directed therapy).6 A retrospective chart review is proposed, in which data collection is to begin at a later date, and that will examine the care provided to patients treated with a new sepsis protocol. The study subjects have not yet received care, but will do so before initiation of data collection. Does the primary investigator need IRB approval? Is informed consent required?
Scenario 2: A randomized controlled study comparing 2 antibiotic regimens for group A streptococcal pharyngitis in a pediatric population, aged 3 to 18 years. In one case, the subject would take medication for 10 days, while in the other, the active medication would be taken for 4 days, followed by 6 days of taking a matching placebo to maintain blinding. Assuming written informed consent is obtained from the parents, would this study be acceptable to the IRB? Would child assent be necessary?
Scenario 3: A randomized controlled study comparing diluted vaccine or placebo to determine effectiveness of smallpox vaccination in a pediatric population. The description of the trial noted that smallpox was currently thought to be eradicated in the natural setting, though stocks of virus may exist for use as a biologic warfare agent. Would the study be acceptable to the IRB? How does the IRB perception that smallpox has been eradicated influence such a decision?
Scenario 4: A randomized controlled trial of therapeutic hypothermia following cardiac arrest in which patients receive either external cooling or an indwelling device. Since all subjects are comatose after arrest, and cooling must be instituted within 20 minutes after resuscitation, would consent be necessary? Who would be consenting?
Study Subjects
The study subjects were a convenience sample of members of the IRB from the UCLA Clinical and Translational Science Institute and its affiliated institutions, as well as from major institutions in South Korea (Table 1). Each IRB member (chairs/vice chairs) was contacted by a personal phone call and via e-mail with a formal invitation letter, consent form, and survey. If there was no response after 2 to 3 weeks, another e-mail reminder was sent. All participating respondents received a 10-dollar gift card. Since the identity of the IRB member was known, the survey response was not anonymous, though the respondents were assured that the results would be deidentified and reported in aggregate.
Data Analysis
All free-text survey responses were carefully reviewed after translation, if indicated. The text was searched for recurrent themes by performing recursive abstraction, which is a technique in which an iterative approach is used to summarize the data.
Numerical data and dichotomous survey responses (yes/no) were also entered into a Microsoft Excel 2003 (Seattle, Washington) spreadsheet, and Database Management Systems Copy (DataFlux Corporation, Cary, North Carolina) was used to convert the file into an SAS version 9.2 (Cary, North Carolina) database. Simple descriptive statistics (eg, the median number of years of IRB experience among respondents and simple proportions or percentages) were tabulated. A priori, we determined that formal hypothesis testing would not be indicated, as this would primarily be a qualitative description of the survey responses.
RESULTS
A total of 22 IRB members from South Korea and 23 IRB members from the United States were contacted. Twenty-three of the 45 survey recipients completed the survey, for an overall response rate of 51%. The median number of years served on an IRB for the total group was 5 (interquartile range [IQR], 3–12). The median number of years served on the IRB was 10.5 (IQR, 5.5–14) for the US cohort versus 3 years (IQR, 0–7) for the Korean cohort. None of the Korean responders were US trained or had served on a US IRB.
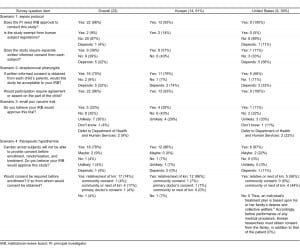
Regarding the sepsis study scenario, only 1 individual, a Korean researcher, stated that IRB approval was unnecessary. Two respondents, both Korean, felt that the sepsis study should be exempt from human subjects’ regulations, if the information was deidentified (“the information of subjects will be recorded in unrecognizable way, like encryption”), whereas 1 US participant stated that exemption would “depend upon the data being collected.” Overall, there was great variability regarding whether informed consent would be required. When comparing the 2 cohorts, most members of the Korean cohort (57%) believed that consent should always be obtained, whereas only 1 US respondent felt that consent should unqualifiedly be obtained. Three US respondents (33%) (versus 6 Korean respondents [43%]) did not believe that consent was needed. The 3 US respondents cited “impracticability” of obtaining informed consent under this circumstance, whereas the 6 Korean respondents mentioned the “retrospective” nature of the data collection and the belief that the Rivers protocol was “standard of care.” Five of the 9 US respondents (56%) stated that whether or not consent would be required would depend upon the circumstances. All 5 of these US respondents, who qualified the answer, stated that waiver of informed consent would be possible under the code of federal regulations, while 2 also mentioned potential “impracticability” of consent due to the patient’s critical status.
Scenario 2 involved the comparison of 2 different antibiotic regimens for the treatment of pediatric streptococcal pharyngitis: one that was 4 days versus another that was 10 days in duration. There was no mention in the scenario as to what duration of treatment was considered standard of care. Overall, 16 respondents (70%) stated that the protocol would be acceptable to their IRB, whereas 2 (9%) stated that it would not, and 5 (22%) qualified that it would “depend” upon the circumstance. When stratifying by country, among the Korean cohort, 11 (79%) reported IRB acceptability, 1 (7%) did not, and 2 (14%) stated that it would “depend.” Of the US respondents, 5 (56%) reported IRB acceptability, 1 (11%) did not, and 3 (33%) stated that it would “depend” upon the circumstances. The reason provided by the US individual who reported unacceptability to the IRB was that “the research does not address the major issue, which is patient compliance. Each (antibiotic) course is still 10 days.” In response to the question of necessity of obtaining pediatric assent, all 9 US participants (100%) affirmed its need, while 13 of the Korean participants (93%) believed that assent was necessary. The 1 Korean subject that reported that assent was not necessary stated that “[c]hildren’s consent is not required.”
Scenario 3, involving a pediatric smallpox vaccine trial, demonstrated a wide range of answers. Overall, 5 (22%) stated that it would be acceptable to the IRB, while 8 (35%) did not think it would be acceptable, and 7 (30%) thought it would be unlikely that the IRB would approve of it. Two US participants (22%) stated that the “IRB would likely refer this trial to the Department of Health and Human Services (DHHS) under child risk category 45 CFR §46.407.” One US participant (11%) and 4 Korean participants (29%) believed that the IRB would approve the study. This US participant stated that since the study involved a “vulnerable population, assurance that the risk-benefit ratio was favorable” would have to first be provided. Three of the 4 Korean participants who stated that their IRB would approve provided no reason for why the protocol would be favorably reviewed; however, 1 stated that “since risks could offer important knowledge for children, benefits are expected and would likely be approved.” Six Korean participants (43%) versus 2 US participants (22%) did not believe that their respective IRB would approve the study. The reasons cited by the 2 US participants were that the “risks outweighed the benefits,” and that there was “insufficient information to approve” the study. Similarly, the 6 Korean participants who did not believe that their IRB would approve cited the fact that smallpox is eradicated, thereby making the risks outweigh any benefit.
For the hypothermia protocol, 78% (18 of 23) of the total cohort stated that their IRB would approve the study. Stratified by country, 86% (12 of 14) of the Korean cohort believed that their IRB would approve, versus 67% (6 of 9) of the US participants. The 1 Korean who stated that their IRB would not approve did not provide a reason, while another Korean respondent who stated that approval “depended” provided several stipulations before approval: “benefits or complications of hypothermia first be described and reviewed…and scientific evidence comparing external cooling with indwelling device should be offered.” Overall, 22 participants (96%) believed that consent was required, whether it was obtained from next of kin (74%), the community (4%), community or next of kin (17%), or the primary doctor caring for the patient. Of the 14 Korean respondents, 13 (93%) reported that consent was required, either from family or next of kin (12 of 14 or 86%), from the community (1 of 14 or 7%), or from the hospital or primary doctor (1 of 14 or 7%). Consent from the community is acknowledged and accepted only after discussion in several public forums. The 1 Korean member who stated that consent was unnecessary did not provide a reason. All 9 of the US respondents stated that consent was required, either from next of kin or from the community.
Although our objective was not to perform any quantitative statistical testing, we found no notable differences in proportions between groups (Korean vs the US IRB members) in any of the survey item responses. However, there were several comments written by individuals that were indicative of cultural and philosophic differences. The themes that emerged after recursive abstraction are described below (Table 3).

Theme 1: Emphasis upon Parental Authority over a Child’s Autonomy
This was a theme that was predominant in the Korean responses. Direct quotations from the Korean respondents include the following: “Children’s consents are not required” [as long as parental consent was obtained]; “IRB can allow for a waiver of assent if the child is less than 13 years of age”; and “after children become adults, they can make the decision to get vaccinated or not to get vaccinated.”
Theme 2: Importance of Child’s Autonomy
In contrast to the Korean emphasis upon parental authority, the US IRB members appeared to emphasize the importance of a child’s autonomy, and his or her ability to provide assent at a younger age: “Assent of the child would be required…for mature teenagers, the investigator may use the adult informed consent form”; “our IRB would require child assent if the child possesses the cognitive capacity to understand the assent process”; “for ages greater than 7, assent of the child would be required; for youths aged 13–18, we would use a youth assent form”; and “assent would be required in this study, which provides no major benefit to the child.” According to several US participants, child assent is necessary for persons older than 7 years in the United States, whereas most Korean participants stated that the age recommended for assent was 13 (neither consent nor assent was viewed to be necessary below age 13).
Theme 3: Importance of Family Who Needs an Experimental Treatment
Both Koreans and US respondents recognized the rights of a proxy or surrogate who can represent individual patient values. Direct quotations from US respondents include: “It is possible that other surrogate consent processes could be used”; “Family members could be consulted”; “consent would be required from a family member”; “a legally authorized representative can give consent—a spouse, adult children, parents, or other family members”; “family members or someone with a durable power of attorney would consent”; and “subject’s family members are approached in a descending order of relationship, specified by the state.”
Direct quotations from the Korean respondents include the following: “the researcher must obtain consent from the family member, as well as the hospital or primary doctor”; “consent should be obtained from the family”; and “legally authorized representative’s consent is required.” In Korea, consent is expected from not just the individual, but also from the physician who is caring for the patient at the time the research is conducted. In the context of medical practice, a Korean hospitalist or emergency physician is empowered and expected to provide input into decisions regarding consent for procedures and research on behalf of the patients, despite the likelihood that the patients may never have met these care providers before this encounter. In the United States, while a researcher would be unlikely to enroll and consent a patient without first speaking to the physician caring for the patient, the physician would not necessarily be expected to sign a written consent on behalf of the patient.
Theme 4: Emphasis upon Greater Good for the Group/Community over the Individual
This was a theme that was apparent among the Korean responses, as seen in the following excerpted quotations: “there are more risks than benefits to each child, but because those risks may offer important knowledge to a great number of children, benefits are expected”; “the benefit of this study is that it could protect the public’s health in advance of smallpox being used as a weapon of terror”; “[whether the IRB would approve the vaccine] depends on the potential morbidity of smallpox to our country. If the morbidity is predicted to be high, then the IRB would approve it”; “the vaccine may benefit society, which will benefit children”; “the attitudes of the community should be considered during the review process”; and “if a legally authorized representative is not available at the time that consent is needed, then the researchers could enroll the patient but obtain consent at a later date.”
Theme 5: Individualism
More notably, the theme of individualism emerged from the US IRB members: “If any data can identify the individual, then it is unlikely to qualify for exemption”; “private information used is not exempt”; “if the Rivers protocol is considered standard of care, then the rights and welfare of the (individual) subject is not harmed. … Requiring consent… could even harm (the individual) subject by delaying their treatment…”; “[The study is unlikely to be approved because it] “is unlikely to provide any benefit to an individual child”; “since there is no known benefit to the individual child, and the case for societal benefit is rather slim…”; “there are no benefits to the individual participants”; and the IRB should assess “the risk of encephalitis versus a potential benefit of protection from a terrorist act for the individual subject.”
… Requiring consent… could even harm (the individual) subject by delaying their treatment…”; “[The study is unlikely to be approved because it] “is unlikely to provide any benefit to an individual child”; “since there is no known benefit to the individual child, and the case for societal benefit is rather slim…”; “there are no benefits to the individual participants”; and the IRB should assess “the risk of encephalitis versus a potential benefit of protection from a terrorist act for the individual subject.”
DISCUSSION
Given the well-known cultural differences between Korea and the United States, between-group differences in the IRB approach to research may have been expected. Yet, there were no clear apparent between-group differences in the dichotomous “yes/no” responses to the survey items. However, no sample size calculation was performed, and our study was not powered to detect any quantitative differences. Moreover, thematic differences emerged when analyzing the free-text responses. While the Korean respondents appeared to emphasize the importance of parental authority and benefiting the community, as well as the importance of the physician’s judgment, the US respondents stressed the autonomy of the child and individualism. The one theme that was common to both US and Korean respondents was the recognition of the rights of a proxy or surrogate who can represent the individual’s values.
Individualism is a core value in American culture; in fact, the US Constitution and the Bill of Rights are based upon the principle that government exists to protect individual rights.7 Respect for person is paramount in the Belmont Report, and this theme of the importance of the individual clearly emerged from the US participants’ survey responses. In contrast, based upon Confucian principles,8,9 Korean collectivism serves as a contrast to the “fundamental American ideology of individualism.” Thus, in Korea, an individual’s treatment plan is based upon his or her family’s desires and collective welfare.8 Accordingly, before performance of any medical procedure, Korean researchers must obtain consent from the family, in addition to that of the patient. The ultimate goal of Confucianism is to achieve social harmony, and to achieve such social harmony, individuals must understand a social order based upon 5 core relationships: ruler to subject, father to son, husband to wife, elder brother to younger brother, and friend to friend. According to Confucian philosophy, individuals are primarily viewed in the context of relationships. For example, a ruler exists to take care of his subjects, whereas a subject exists to follow a ruler’s commands. The hierarchy in social and family relationships is also distinct in Confucian beliefs. Knowing this philosophic background helps in understanding how a Korean researcher may approach child assent, a community trial, and a subject’s family members.
While Western cultures are obviously not based upon Confucian philosophy, Western Judeo-Christian religions clearly stress the importance of family relationships, as well. Thus, it is not too surprising that the family unit emerged as an important theme among both Korean and US respondents.
LIMITATIONS
Our study has several limitations. First, we had only 23 respondents. Our survey response rate was 51%, and our results are thus subject to a self-selection bias. There were also proportionally more US nonrespondents than Korean nonrespondents, and we were unable to identify the reasons for nonresponse. It is unlikely, however, that respondents would systematically differ from nonrespondents such that our qualitative analysis would differ. For example, there is little reason to believe that those who emphasize child autonomy would be less likely to respond than those who view child autonomy as a paramount ethical virtue.
Second, the Korean respondents had served on an IRB for a significantly fewer number of years. It is possible that with increasing experience, the Korean IRB members would become more “westernized” in their thought processes and increasingly emphasize the importance of individual autonomy and respect for persons. However, it is unknown how more experience would have influenced a Korean IRB member.
Third, there is the possibility of social desirability bias. As mentioned previously, the Korean government established the KAIRB to help the Korean IRBs meet international medical ethics standards. Korean IRB members therefore most likely know what is written in the Belmont Report, and the respondents may wish to appear to conform to what is perceived to be the “correct” answer. In the absence of social desirability bias, the respective differences in how each IRB views the individual, the child, the family physician, and the community may have been clearer.
Finally, although the survey instrument was translated into Korean by a bilingual medical professional, and both Korean and English versions of the survey were administered to the Korean respondents, the survey has not been validated and it is possible that the respondents did not fully understand the questions.
CONCLUSION
Whereas the US survey respondents appear to emphasize the importance of the individual and the autonomy of a child, the Korean respondents stressed the importance of parental authority and benefiting the community, above and beyond that of the individual person. However, there was substantial overlap in the themes expressed by respondents from both countries. Still, while the interpretation of a given research project may depend upon cultural norms and mores, every research project should nonetheless be ethically sound.
Footnotes
Supervising Section Editor: Eric R. Snoey, MD
Reprints available through open access at http://escholarship.org/uc/uciem_westjem
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding, sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
Address for correspondence
Roger J. Lewis, MD, PhD
Harbor-UCLA Medical Center, Department of Emergency Medicine
Building D9, 1000 W Carson St, Box 21, Torrance, CA 90509-2910
E-mail: roger@emedharbor.edu
REFERENCES
1. Nuremberg Code. Available at: http://ohsr.od.nih.gov/guidelines/nuremberg.html. Accessed January 15, 2010.
2. Enfield KY, Truwit JD. The purpose, composition, and function of an Institutional Review Board: balancing priorities. Respir Care. 2008;53:10–14.
3. The Belmont Report Ethical Principles and Guidelines for the protection of human subjects of research. Available at: http://ohsr.od.nih.gov/guidelines/belmont.html. Accessed January 15, 2010.
4. Lee J, Kim O, Kim S, et al. Current status and problems of Institutional Review Boards in Korea.Korean J Med Ethics. 2006;9:203–222.
5. Shin SG. The current status of clinical trials in the Republic of Korea. Drug Inf J. 1998;32:S1217–S1222.
6. Rivers E, Ngueyn B, Harstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. [PubMed]
7. Bill of Rights. Available at:http://www.archives.gov/exhibits/charters/bill_of_rights_transcript.html. Accessed January 15, 2010.
8. Lee SM. A cross-cultural approach to biomedical ethics: medical decision making. Korean J Med Ethics Educ. 2007;10:23–32.
9. Ko E, Lee J. End-of-life communication: ethnic differences between Korean and American and non-hispanic white older adults. J Aging Health. 2009;21:967–984. [PubMed]


