| Author | Affiliation |
|---|---|
| Laleh Gharahbaghian, MD | Stanford University Medical Center, Division of Emergency Medicine, Palo Alto, CA |
| Bobby Massoudian, MD, MS | Long Beach Memorial Medical Center, Department of Emergency Medicine |
| Giancarlo DiMassa, MD, MSHS | Long Beach Memorial Medical Center, Department of Emergency Medicine |
ABSTRACT
This case report describes two pediatric cases of immediate oxygen desaturation from methemoglobinemia and sulfhemoglobinemia after one sip from a plastic water bottle containing hydroxylamine sulfate used by a relative to clean shoes. Supplemental oxygen and two separate doses of methylene blue given to one of the patients had no effect on clinical symptoms or pulse oximetry. The patients were admitted to the pediatric Intensive Care Unit (ICU) with subsequent improvement after exchange transfusion. Endoscopy showed ulcer formation in one case and sucralafate was initiated; both patients were discharged after a one-week hospital stay.
INTRODUCTION
Sulfhemoglobinemia is a rare condition that can result from exposure to any substance containing a sulfur atom with the ability to bind to hemoglobin. Cases of sulfhemoglobinemia have been reported from ingestions of phenacetin, dapsone, and sulfonamides.1–3 Sulfhemoglobinemia should be considered in cases presenting with oxygen desaturation and cyanosis, especially if methemoglobinemia can be excluded. Unlike methemoglobinemia, which is reversible with a known antidote, methylene blue, sulfhemoglobinemia is irreversible with no known antidote. It requires early recognition, diagnosis and intervention in order to prevent end-organ damage, especially with high levels of sulfhemoglobin. The irreversibility of sulfhemoglobinemia illustrates the importance of its consideration as a diagnosis in the emergency department (ED).3 Using PubMed and Medline search engines from the National Library of Medicine, as well as the MD Consult website, we conducted a review of all-language medical literature from January 1966 to August 2008 using the search parameters “Sulfhemoglobinemia and/or Methemoglobinemia and Hydroxylamine Sulfate.” The search resulted in zero articles. We also searched using Medical Subject Headings (MeSH) terms “pediatrics” and “sulfhemoglobinemia.” This generated a list of one article, a review.4 Finally, we reviewed the bibliography of the review found in the Medline search and looked for any obvious omissions from our literature review. Based on our literature review, we believe this to be the first report of pediatric patients developing methemoglobinemia and sulfhemoglobinemia from the ingestion of hydroxylamine sulfate.
CASE 1
A three-year-old male was brought by paramedics to the ED accompanied by his mother. She stated he had immediate blue-gray discoloration of skin, increased somnolence and abdominal pain after drinking one sip from a water bottle found in his sister’s room. The bottle, brought in by the paramedics, was noted to contain a colorless fluid. The patient’s mother denied knowledge of any substance mixed with the water and any toxic substances or drugs in the home.
After the three-year-old drank from the bottle, he then gave it to his two-year-old cousin whose findings are discussed below. On history, the three-year-old boy pointed to his epigastrium for the location of the abdominal pain. He stated that the severity was “a little” and the quality was “sharp.” There was no vomiting, diarrhea, cough, change in behavior, or loss of consciousness. The patient was not taking medications, and past medical and family histories were unremarkable.
The physical exam showed an oral temperature of 36.4 degrees Celsius, heart rate of 120 beats/minute, respiratory rate of 30 breaths/min, blood pressure of 115/43 mmHg, and oxygen saturation on room air of 84% by pulse oximetry. The patient weighed 18.5 kg. He demonstrated central cyanosis. Otherwise, his general appearance was of an alert and oriented male in no apparent distress. He was not diaphoretic, his mucous membranes were moist, his bowel sounds were normal, and his neurologic exam was normal.
An arterial blood gas (ABG) while the patient was on 100% oxygen via non-rebreather face mask showed a pH of 7.53 (reference range 7.35–7.42), pCO2 of 25.2 mmHg (36–50), pO2 of 249 mmHg (80–100), and HCO3 21.2 mmol/L (24–29). His calculated oxygen saturation on arterial blood gas (ABG) was 100%, whereas the finger pulse oximeter showed 84% oxygen saturation. Laboratory studies included a complete metabolic panel and complete blood count (Table).
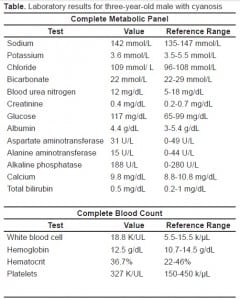
Ethanol level was less than 10 mg/dL. A midstream clean-catch urinalysis was normal, and a rapid urine drug screen was negative for amphetamines, barbiturates, benzodiazepines, opiates, cocaines, phencyclidines, and cannabanoids. The carboxyhemoglobin, methemoglobin and sulfhemoglobin levels had to be sent to an outside hospital lab. A chest radiograph showed no evidence of acute disease.
The three-year-old patient was placed on 100% supplemental oxygen and cardiac monitor, which showed normal sinus rhythm. An intravenous line (IV) was placed. The social worker attempted to locate the patient’s sister who had the bottle in her room. The Poison Control Center recommended giving a dose of methylene blue at a dose of 1mg/kg. Methylene blue had no effect. At that time the blood specimen originally sent for analysis of the methemoglobin level was noted to be hemolyzed, and a second specimen was sent to the lab. A second dose of methylene blue at 1mg/kg was administered without effect.
The patient’s older sister, who then arrived at the ED, stated that she had put a substance to clean shoes into the water bottle. It was “HAS”: hydroxylamine sulfate. At the same time the patient’s carboxyhemoglobin level returned from the lab at 0.6% (0%–5%) and methemoglobin level was 5.4% (0.4%–1.5%). He was admitted to the pediatric ICU.
The Gastroenterology service performed endoscopy on the patient due to continued abdominal pain. Endoscopy revealed two 1.5 cm by 1.5 cm Grade II ulcers (through the mucosa and muscularis mucosa) in the upper esophagus and multiple small, superficial erosions in the mid-esophagus, none of which were circumferential. The patient was started on sucralafate and ranitidine.
The patient’s methemoglobin levels improved gradually after admission, dropping to 4.5% after the two doses of methylene blue described above. At 24 hours the level was 3.1%, and by 36 hours post-ingestion hismethemoglobin level had fallen to 2.7%. At that time, Hematology was consulted due to continued low oxygen saturations despite lowering methemoglobin levels. Hematology recommended partial exchange transfusion with packed red blood cells and fresh frozen plasma. Prior to the initial exchange transfusion, the patient’s oxygen saturation was 85% by pulse oximetry while receiving supplemental oxygen via a non-rebreather face mask. The exchange transfusion had to be stopped as he developed hives on his face and chin, but more than three quarters of a unit of blood and a single unit of fresh frozen plasma was administered. The resultant oxygen saturation was documented in the low 90s. On the third hospital day, packed red blood cells were transfused without exchange, and by day four, his oxygen saturation had improved to 94%. The patient was discharged home on the fifth hospital day with stable vital signs (including oxygen saturation of 97%) and without end organ damage. The initial sulfhemoglobin level, which was sent on the day of the patient’s arrival, returned 10 days after patient’s discharge and was noted to be 10 % (0–2%).
CASE 2
A two-year-old female accompanied the above patient. She also had blue-gray discoloration after drinking one sip from the plastic water bottle and complained of similar abdominal pain. There was no somnolence, nausea, vomiting, change in behavior, or loss of consciousness. The past medical and family histories were unremarkable, and she was not taking any medications.
The physical exam showed an oral temperature of 36.7 degrees Celsius, heart rate 120 beats/min, respiratory rate 26 breaths/min, capillary refill of less than 2 seconds, and oxygen saturation of 84% on room air by pulse oximetry. The patient’s weight was 15 kg. She was noted to be alert and oriented, in no apparent distress, speaking appropriately, with blue-gray central skin discoloration. The remainder of the physical exam was normal including clear lung sounds without stridor.
An immediate blood gas showed a pH 7.45, pCO2 28 mmHg, pO2 233 mmHg, HCO3 19.5 mmol/L and oxygen saturation of 100% while the finger pulse oximeter showed an oxygen saturation of 92% on 100% supplemental oxygen via mask. Laboratory studies included a complete metabolic panel that was within normal limits. A complete blood count showed white blood cell 9.7 K/UL, hemoglobin 10 g/dL, hematocrit 29.4%, platelets 301 K/UL. Ethanol level was <10 mg/dL, and a urinalysis and urine drug screen were negative. A chest radiograph showed no evidence of acute disease.
The same management was taken as with the previous patient. After the methemoglobin level was tested to be 3.8% with a normal carboxyhemoglobin level at 1.3%, methylene blue was administered at 1mg/kg without effect prior to admitting the patient to the pediatric ICU.
Gastroenterology performed an endoscopy showing no ulcers. On the second hospital day, hematology recommended packed red blood cell transfusion due to hemoglobin falling to 9.1 g/dL due to presumed hemolysis from oxidant stress from the toxin. This improved her oxygen saturations. Exchange transfusion was not recommended, as she was asymptomatic. The patient was weaned off supplemental oxygen and discharged home on the sixth hospital day.
DISCUSSION
These cases describe patients who presented with acute oxygen desaturation via pulse oximetry and central cyanosis after drinking one sip of water mixed with hydroxylamine sulfate, which resulted in methemoglobinemia and sulfhemoglobinemia, a rare combination.
Hydroxylamine sulfate is a strong acid and powerful reducing agent. It is a white crystalline compound containing nitrogen and sulfate with the formula of (NH2OH)2H2SO4 that can cause irritation to the nose and throat, as well as pulmonary edema if inhaled.4 It is also corrosive and can cause burns to skin and, if ingested, in the mouth, esophagus and stomach, as was evident in one of our patients. It is known to cause methemoglobinemia, but sulfhemoglobinemia is not a stated result of exposure in the Material Safety Data Sheet (MSDS).5 It is usually used in photography and surface cleaning solutions.5 Our patient’s older sister obtained the product from their mother’s work, which has housecleaning products. It was mixed with water in a plastic bottle, was not labeled and easily accessible.
As with all cases of acute oxygen desaturation, a search for the etiology is emergently required. After mechanical obstruction and cardiac shunting has been eliminated as a possibility, ventilation problems such as pneumothorax, pneumonia, or asthma and perfusion problems, such as pulmonary embolism, should be considered. Once these problems have been sufficiently ruled out, evaluation for abnormal blood hemoglobin is warranted.6,7 The “oxygen saturation gap” is the difference between the calculated oxygen saturation from a standard blood gas machine and the reading from a pulse oximeter. If it is greater than 5%, the patient’s hemoglobin may be abnormal, representing carbon monoxide poisoning, methemoglobinemia, or sulfhemoglobinemia. Our patients had no symptoms of mechanical obstruction, no history of reactive airway disease or cardiac abnormalities or fever, and no risk factors for pulmonary embolism. Their acute oxygen desaturation event occurred immediately after ingestion of the colorless fluid in the water bottle. Although the chemical was not known at the time, the suspicion for a toxic ingestion was high.
Methemoglobin is a product of hemoglobin in which the normal ferrous ion in the heme complex is converted by oxidation to the ferric form which does not combine with oxygen, but can convert back to hemoglobin by reducing agents such as methylene blue.7–11Acquired methemoglobinemia is produced by the action of oxidants.12 This leads to a leftward shift of the oxygen dissociation curve for the remaining normal hemoglobin, resulting in diminished oxygen unloading in the tissues and predisposing to tissue hypoxia.7–10
Several enzymes work to decrease the amount of circulating methemoglobin molecules. Cytochrome b5 reductase is the primary enzyme that works to decrease levels of oxidized hemoglobin (such as methemoglobin) by reducing the molecule. Other enzymes include glutathione peroxidase and catalase. Cellular hypoxia can occur if an abnormally increased oxidant stress exceeds the normal source of reducing power. Methylene blue is used as an electron donor in chemical-induced methemoglobinemia, which utilizes NADPH and the hexose monophosphate pathway to reduce methemoglobin to hemoglobin. This reduction occurs quickly over several minutes. The regeneration of NADPH requires an intact pentose phosphate pathway. It is also critical to remember that in those patients with glucose-6-phosphate dehydrogenase deficiency, methylene blue has no effect and can actually induce acute hemolysis.10,11
Sulfhemoglobin is a stable, green-pigmented molecule, which is not normally present in the body. It is made by the oxidation of the iron in hemoglobin to a ferric state by drugs and chemicals that contain sulfur. Sulfur can bind to the hemoglobin molecule’s porphyrin ring, which forms sulfhemoglobin. Sulfhemoglobin is irreversible, lasting the lifetime of the erythrocyte, and sulfhemoglobin molecules cannot carry oxygen.3,9,12,13Acetanilide, phenacetin, nitrates, trinitroluene, metoclopramide, and sulfur compounds have all been linked to producing sulfhemoglobinemia. The origin of sulfur, in cases where it is not overtly apparent, has been theorized to come from hydrogen sulfide released by intestinal organisms and/or glutathione.3,7,9,13
The concentration of sulfhemoglobin decreases as erythrocytes are destroyed and replaced.3 The decreased oxygen affinity of the unaffected hemoglobin results in protection of tissue oxygen delivery. Sulfhemoglobinemia is a rare cause of cyanosis, and patients present with mild to moderate clinical symptoms.3,10,13 Dyspnea is uncommon unless the level of sulfhemoglobin is high, although cyanosis can set in at much lower concentrations. It is reported that levels of only 0.5g/dL is sufficient for cyanosis to occur.9,10,11 Our patients denied any symptoms consistent with end-organ damage (including dyspnea), but had obvious cyanosis, which in and of itself is not an indicator of tissue hypoxia.
Reports of physiologic affects of sulfhemoglbinemia display little consistency.6,9,14,15While some describe sulfhemoglobin levels of 20% to 60% as benign for some patients, these lack evaluation of tissue oxygen status or of its influence on the course of patient outcome in the face of cardiac and pulmonary involvement.6,15
The diagnosis of true sulfhemoglobinemia can be difficult. Any time there is a significant pulse oximetry desaturation associated with a normal arterial oxygen tension (PaO2) or anytime a patient remains cyanotic without response to methylene blue, an emergency physician should consider the possibility of abnormal hemoglobin species interfering with the pulse oximeter other than methemoglobin.3,13 Our patients illustrated this point clearly since there was no effect in oxygen saturation after methylene blue.
The knowledge of the type of co-oximeter used in the analysis of ABGs is also essential. Hemoximeters use multiple wavelengths to determine concentrations of oxyhemoglobin, deoxyhemoglobin, carboxyhemoglobin, and methemoglobin. However, different co-oximeters vary widely, with some not being able to distinguish between methemoglobin and sulfhemoglobin due to similar absorbance peaks. Gas chromatography is considered the “criterion” standard. However, to perform gas chromatography, specialized equipment, time and expertise are required.9,16
Some pulse oximeters use two light wavelengths (660nm and 940nm) to determine the ratio of pulse-added absorbancies. Dyshemoglobin molecules that have light absorbance peaks at 660nm or 940nm affect the ratio of light absorbancies at these wavelengths and lead to spurious pO2 readings. Methemoglobin has significant absorbancies at both wavelengths. Sulfhemoglobin molecules share a similar peak with methemoglobin molecules at 630nm. Therefore, a reported methemoglobin level may actually be sulfhemoglobin and be inappropriately treated with methylene blue as has been described in other case reports.9 Further complicating the clinical picture, some substances can cause both methemoglobin and sulfhemoglobin.9,10,11 The blood gas analyzer at the outside institution for our case was able to accurately differentiate methemoglobinemia from sulfhemoglobinemia.
Sulfhemoglobinemia may be distinguished from methemoglobinemia by isoelectric focusing.4 The laboratory measurement of sulfhemoglobin relies on an absorption peak at 630nm, which, unlike methemoglobin, persists after the addition of cyanide or dithionate.3,7,12 Another method of differentiating sulfhemoglobin from methemoglobin involves carbon monoxide, since it binds to sulfhemoglobin but not to methemoglobin. Finally, newer generation co-oximeters that are designed to assess sulfhemoglobin and other types of hemoglobin can differentiate between the two types.9,17,18 The sulfhemoglobin level in our case returned 10 days after initially sent and was analyzed in an outside laboratory using potassium cyanide.
There is no specific treatment for sulfhemoglobinemia. Most treatment recommendations for sulfhemoglobinemia state to remove the offending agent, and with low levels of sulfhemoglobin, no more than observation is needed, usually until they are clinically stable and it is clear that the cyanosis and/or sulfhemoglobin level is improving.3,6,9,11,15Lim and Lower19 suggest exchange transfusion as a means of managing extreme sulfhemoglobinemia. Other sources state that exchange transfusion is hardly justified, since the cyanosis itself is in no way disabling.12 Exchange transfusion and packed red blood cell transfusion improved oxygen saturations in our patients, reversed the cyanosis, and maintained normal oxygen saturation through the rest of their hospital stay. In retrospect, these interventions may not have been necessary in our patients, since low levels sulfhemoglobin will cause little if any effect. Also, exchange shares similar risks as other blood transfusion products.
CONCLUSION
Hydroxylamine sulfate, a strong acid known to cause methemoglobinemia but not sulfhemoglobinemia, was mixed in a water bottle and not appropriately labeled. The hydroxylamine sulfate was ingested by two children causing a mixed methemoglobinemia and sulfhemoglobinemia. Sulfhemoglobinemia itself is rare, difficult to distinguish from methemoglobinemia, and (at high levels) can result in end-organ damage. Our patients did not respond to methylene blue treatment, illustrating that their oxygen desaturation was mainly due to sulfhemoglobinemia. Sulfhemoglobinemia may appear identical to methemoglobinemia by co-oximeters that cannot differentiate between the two molecules. Knowledge of the co-oximeter used at your institution can help to differentiate the abnormal hemoglobin molecules. Exchange transfusion and packed red blood cell transfusion immediately increased our patients’ oxygen saturations, which may have prevented end-organ damage, illustrating the importance of early recognition and intervention. However, given the low levels of sulfhemoglobin, observation alone may have been sufficient.
Footnotes
We would like to thank Chris Babbit MD, for his excellent work caring for the patients described in the manuscript in the Pediatric Intensive Care Unit at Miller Children’s Hospital at Long Beach Memorial and for his help with follow-up.
Supervising Section Editor: Jeffrey R. Suchard, MD
Submission history: Submitted September 14, 2008; Revision Received January 30, 2009; Accepted February 02, 2009
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Address for Correspondence: Giancarlo DiMassa, MD, MSHS, Long Beach Memorial Medical Center, Emergency Department, 2801 Atlantic Avenue, Long Beach, CA 90801
Email: gdimassa@gmail.com
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Schmitter CR. Sulfhemoglobinemia and methemoglobinemia: Uncommon causes of cyanosis. Anesthesiology. 1975;43:586–7. [PubMed]
2. Aravindhan N, Chisholm DG. Sulfhemoglobinemia presenting as pulse oximetry desaturations. Anesthesiology. 2000;93:883–884. [PubMed]
3. Park CM, Nagel RL. Sulfhemoglobinemia. New Engl J Med. 1984;310:1579–84.[PubMed]
4. Mack E. Focus on diagnosis: co-oximetry. Pediatr Rev. 2007;28:73–4. [PubMed]
5. Science Stuff, Inc MSDS (Material Safety Data Sheet) 2006: Hydroxylamine. Sciencestuff.com Web site. Available at: http://www.sciencestuff.com/msds/C1889.htmlAccessed January 5, 2009.
6. Beutler E. Methemoglobinemia and sulfhemoglobinemia. In: Williams WJ, Beutler E, Erslev AJ, Lichtman MA, editors. Hematology. 4th ed. New York NY: McGraw-Hill; 1990. pp. 743–745.
7. Brandenberg RO, Smith HL. Sulfhemoglobinemia; a study of 62 clinical cases. Am Heart J. 1951;42:582–8. [PubMed]
8. Finch CA. Methemoglobinemia and sulfhemoglobinemia. New Engl J Med.1948;239:470–8. [PubMed]
9. Lu HC, Shih RD, Marcus S, Ruck B, Jennis T. Pseudomethemoglobinemia. Arch Pediatr Adolesc Med. 1998;152:803–5. [PubMed]
10. Noor M, Beutler E. Acquired sulfhemoglobinemia. An underreported diagnosis? West J Med. 1998;169:386–389. [PMC free article] [PubMed]
11. Rosen PJ, Johnson C, McGehee WG, Beutler E. Failure of methylene blue treatment in toxic methemoglobinemia. Association with glucose-6-phosphate dehydrogenase deficiency. Ann Intern Med. 1971;75:83–86. [PubMed]
12. Fichter EG. Sulfhemoglobinemia. Am J Dis Child. 1954;88:749–53.
13. Bowie VL, Bharadwaj P. Phenazopyridine induced sulfhemoglobinemia. Ann Pharmacother. 2005;39:1128–1130. [PubMed]
14. Langford SL, Sheikh S. An adolescent case of sulfhemoglobinemia associated with high-dose metoclopramide and N-acetylcysteine. Ann Emerg Med. 1999;34:538–41.[PubMed]
15. O’Conner JM. Cyanosis, sulfhemoglobinemia: a case report. Respir Care.1993;38:1088–1091.
16. Mahoney JJ, Vreman HJ, Stevenson DK. Measurement of carboxyhemoglobin and total hemoglobin by five specialized spectrophotometers (CO-oximeters) in comparison with reference methods. Clin Chem. 1993;39:1693–1700. [PubMed]
17. Wu C, Kenny M. A case of sulfhemoglobinemia and emergency measurement of sulfhemoglobin with an OSM3 CO-oximeter. Clinical Chemistry. 1997;43:162–166.[PubMed]
18. Demedts P, Wauters A, Watelle M, et al. Pitfalls in discriminating sulfhemoglobin from methemoglobin. Clin Chem. 1997;43:1098–1099. [PubMed]
19. Lim TK, Lower D. “Enterogenous” cyanosis. Am Rev Respir Dis. 1970;101:419–422.[PubMed]


