| Author | Affiliation |
|---|---|
| Heidee Villanueva, MD | Department of Emergency Medicine, Keck School of Medicine of the University of Southern California |
| Marius Tijunelis, MD | Department of Emergency Medicine, Keck School of Medicine of the University of Southern California |
| Sharon Shapiro, BA | Department of Emergency Medicine, Keck School of Medicine of the University of Southern California |
| Sean O. Henderson, MD | Department of Emergency Medicine, Keck School of Medicine of the University of Southern California |
ABSTRACT
A 19-year-old female with Systemic Lupus Erythematosus (SLE) presented with ischemia of her left hand following trauma. Medical therapy was initiated but failed to improve her symptoms, and revision amputation was ultimately performed. The patient’s final diagnosis was digital ischemia due to secondary Raynaud’s Phenomenon (RP). The authors discuss diagnosis, complications, and treatment of this relatively uncommon disorder. The authors report this case in order to discuss how secondary RP can be complicated by ischemia and the multidisciplinary approach that needs to take place to prevent the latter from occurring.
INTRODUCTION
Raynaud’s phenomenon (RP) presents as a challenging disease for Emergency Physicians (EP) due to its variability in presentation, largely variable medical treatments, and risk of significant morbidity. The disease is described as an episodic vasospasm of peripheral arteries, causing pallor, cyanosis, and at times, subsequent ischemia. It was first described by Maurice Raynaud in 1862 as a localized “syncope.”1 Primary RP is idiopathic, and secondary RP occurs in association with an underlying disease, usually a mixed connective tissue disorder (MCTD). The diagnosis is based solely on the clinical presentation since there are no specific tests to detect primary RP. Prevalence is estimated between 3–5% in the general population.2 Despite its high frequency, the majority of available therapies have not been validated in randomized controlled trials (RCT).
CASE REPORT
A 19-year-old female was admitted to the hospital because she had ongoing ischemia to her left index and small fingers. Forty hours before admission, the trauma to her fingertips when she caught her hand in a car door. Thirty-five hours before this presentation, the patient was seen in another emergency room and was noted to have a bluish discoloration to her fingertips with a history of systemic lupus erythematosus. She was diagnosed with RP and given amlodipine and aspirin. A few hours later, without relief of her symptoms and worsening pain, the patient was transferred for higher level of care to a second emergency department.
Upon arrival, the patient was afebrile with stable vital signs, including a temperature of 98°F, blood pressure of 125/92 mmHg, pulse of 113, respiratory rate of 18, O2 saturation of 97% in room air. She was very emotional and anxious and expressed a pain level of 10 out of 10. Her left hand showed a purplish-blue discoloration and edema to the fourth and fifth digits, accompanied by bullae on the ulnar surface of her small finger (Figure 1). She was tender to palpation along the flexor sheath, but both flexor digitorum profundus and superficialis were intact. Pulse oximetry to the affected digits was zero, and she had decreased perception to light touch.
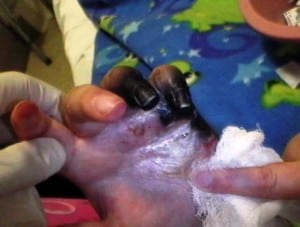
Necrotic fourth and fifth digits, five days after presentation of SLE patient with secondary Raynaud’s phenomenon
The patient was given benzodiazepines and pain medication. Orthopaedics was quickly involved on the case and an angiogram ordered. Rheumatology upon consultation added solumedrol, and the patient was started on an epoprostenol drip.
Left upper extremity angiogram findings included a patent left axillary, brachial, ulnar, radial, deep and superficial palmar arches, common and proper digital arteries 1–3. There was complete occlusion of proper digital arteries for the fourth and fifth digits. There was no response to intra-arterial vasodilator and the patient was deemed an unlikely candidate for lytic therapy secondary to the distal nature of the occlusion.
After five days of epoprostenol drip with minimal improvement in her symptoms, the patient was scheduled for revision amputation. Final pathology diagnosis was necrosis, mixed inflammation consistent with gangrene due to secondary RP.
DISCUSSION
Secondary RP is less common than primary RP and occurs in association with an underlying disease, typically connective tissue, neurovascular, hematological, or drug-induced disorder.3 While primary Raynaud phenomenon (RP) rarely leads to complications, secondary RP, when associated with a mixed connective tissue disease (MCTD), can quickly progress to catastrophic ischemic events resulting in the loss of fingers, toes, and even limbs.4 Timely therapeutic intervention is therefore required. Primary and secondary RP appear to be in a continuum. In one study, as many as 20% of participants followed prospectively were later diagnosed with a MCTD.5 Determining the root cause of a Raynaud crisis can be difficult, and emergent treatment may be challenging due to lack of clearly effective therapy. Therapeutic measures have included a range from avoiding triggers to vasodilator drugs or anti-platelet aggregation drugs to spinal cord stimulation and surgery. Treatment should be individualized for every patient depending on disease severity.
Conservative treatment for RP includes warming and stress reduction,2 however, we found no satisfactory RCTs on the effects of warming. Six small RCTs found that nifedipine reduced the frequency and severity of attacks over 4–12 weeks compared with placebo, and was rated by participants as more effective than placebo in improving overall symptoms. The participants experienced an average of 2.8 to 5.0 fewer attacks per week and a 33% reduction in severity. It was found that nifedipine was associated with higher rates of adverse effects compared with placebo, causing several participants to drop out of every study. Adverse effects include flushing, headache, edema, and tachycardia.6
The use of aspirin and dipyridamole in patients with secondary RP has been studied in at least two controlled trails. Neither demonstrated any significant effect.7,8 Grader-Beck and Wigley recommend that patients with MCTD be placed on anti-platelet therapy with 81 mg of aspirin daily, unless there is a contraindication. They also recommend the use of heparin for 24 to 72 hours for patients who have rapidly advancing ischemic disease, but do not recommend chronic anticoagulation, despite the fact that this has not been studied.2 A recent study demonstrated the benefit of low molecular weight heparin for symptomatic improvement in primary and secondary RP.9
When ischemia progresses rapidly and fails to respond to standard vasodilatory therapy, intravenous iloprost, alprostadil, or epoprostenol may be given.2 Similar results in a meta-analysis were obtained for intravenous infusions of iloprost in patients with secondary RP associated with systemic sclerosis. In addition, intravenous infusions of iloprost improved healing of fingertip ulcers in patients with systemic sclerosis.
Small single randomized controlled trials include angiotensin II-receptor type 1 antagonists (losartan), the calcium channel blockers felodipine and amlodipine, serotonin-reuptake-inhibitors (fluoxetine) and phosphodiesterase-V-inhibitors (sildenafil, vardenafil). However, the results promising substances have to be confirmed in long-term trials with larger patient numbers.10,11,12,13
When medical therapy fails, several surgical interventions can be considered. These include proximal or distal (digital) sympathectomy and arterial reconstruction. Distal sympathectomy is associated with a lower complication rate than proximal sympathectomy, but the long-term outcome has not been well-documented.14,15 As with other vasodilator therapy, sympathectomy is less effective in patients who have secondary RP.2,16 Amputation of digits appears to occur infrequently and is usually reported as individual cases.17,18
If there is evidence of larger vessel occlusive disease, such as has been reported at the level of the ulnar or radial arteries, vascular reconstruction can be performed successfully with vein grafts.4 Therefore, all patients who present with a critical ischemic crisis should have a careful assessment to detect any correctable macrovascular disease. To further define the magnitude of larger vessel disease in these cases, arterial doppler studies, magnetic resonance angiography, or angiography can be used.2
When associated with MCTD, RP requires aggressive diagnostics and initial therapy to decrease arterial vasospasm. Management of this disease requires a multidisciplinary effort in the coordination of a sound treatment plan for without this focus, even minor trauma may result in digital amputation.
Footnotes
Submission history: Submitted May 8, 2007; Accepted November 2, 2007.
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Address for correspondence: Heidee D. Villanueva, DO Department of Emergency Medicine, LAC+USC Medical Center, Unit #1, Room 1011, 1200 N. State St., Los Angeles, CA 90033
Email: Sohender@usc.edu
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Belch JJ. The phenomenon, syndrome and disease of Maurice Raynaud. Br J Rheumatol.1990;29:162–165. [PubMed]
2. Grader-Beck T. Raynaud’s phenomenon in mixed connective tissue disease. Rheum Dis Clin North Am. 2005;31:465–481. [PubMed]
3. Cheng KS, Tiwari A, Boutin A, Denton CP, Black CM, Morris R, Seifalian AM, Hamilton G. Differentiation of primary and secondary Raynaud’s disease by carotid arterial stiffness. Eur J Vasc Endovasc Surg. 2003;25:336–341. [PubMed]
4. Hummers LK, Wigley FM. Management of Raynaud’s phenomenon and digital ischemic lesions in scleroderma. Rheum Dis Clin N Am. 2003;29:293–313.
5. Fitzgerald O. Prospective study of the evolution of Raynaud’s phenomenon. Am J Med.1988;84:78–126.
6. Thompson AE, Pope JE. Calcium channel blockers for primary Raynaud’s phenomenon: a meta-analysis. Rheumatology(Oxford) 2005;44:145–150. [PubMed]
7. Van der Meer J, Wouda AA, Kallenberg CG. A double-blind controlled trial of low dose acetylsalicylic acid and dipyridamole in the treatment of Raynaud’s phenomenon. Vasa Suppl. 1987;18:71–75. [PubMed]
8. Beckett VL, Conn DL, Fuster V. Trial of platelet-inhibiting drug in scleroderma. Double-blind study with dipyridamole and aspirin. Arthritis Rheum. 1984;27:1137–1143.[PubMed]
9. Denton CP, Howell K, Stratton RJ. Long-term low molecular weight heparin therapy for severe Raynaud’s phenomenon: a pilot study. Clin Exp Rheumatol. 2000;18:499–502.[PubMed]
10. La Civita L, Pitaro N, Rossi M, Gambini I, Guiggioli D, Cini G, Ferri C. Amlodipine in the treatment of Raynaud’s phenomenon. A double-blind placebo-controlled crossover study. Clin Drug Invest. 1997;13:126–131.
11. Rhedda A, McCans J, Willan AR, Ford PM. A double blind placebo controlled crossover randomized trial of diltiazem in Raynaud’s phenomenon. J Rheumatol. 1985;12:724–727. [PubMed]
12. Wollersheim H, Thien T, Fennis J, van Elteren P, van’t Laar A. Double-blind, placebo-controlled study of prazoxin in Raynaud’s phenomenon. Clin Pharmacol Ther.1986;40:219–225. [PubMed]
13. Heymann WR. Sildenafil for the treatment of Raynaud’s phenomenon. J Am Acad Dermatol. 2006;55:501–502. [PubMed]
14. Lowell RC, Gloviczki P, Cherry KJ., Jr Cervicothoracic sympathectomy for Raynaud’s syndrome. Int Angiol. 1993;12:168–172. [PubMed]
15. Sayers RD, Jenner RE, Barrie WW. Transthoracic endoscopic sympathectomy for hyperhidrosis and Raynaud’s phenomenon. Eur J Vasc Surg. 1994;8:627–631. [PubMed]
16. Gifford RW, Jr, Hines EA, Jr, Craig WM. Sympathectomy for Raynaud’s phenomenon; follow-up study of 70 women with Raynaud’s disease and 54 women and secondary Raynaud’s phenomenon. Circulation. 1958;17:5–13. [PubMed]
17. Struthers GR, Scott DL, Scott DGI, Bacon PA. Partial finger loss: an indicator of severe connective tissue disease with overlap features. Br J Rheumol. 1983;22:213–217.
18. Landry GJ, Edwards JM, Porter JM. Current management of Raynaud’s syndrome.Adv Surg. 1996;30:333–347. [PubMed]


