| Author | Affiliation |
|---|---|
| Michael Levine, MD | Banner Good Samaritan Medical Center. Department of Medical Toxicology, Phoenix, AZ Banner Good Samaritan Poison Control and Drug Information Center, Phoenix, AZ |
| Josh Canning, MD | Banner Good Samaritan Medical Center. Department of Medical Toxicology, Phoenix, AZ Banner Good Samaritan Poison Control and Drug Information Center, Phoenix, AZ |
| Robyn Chase, DO | Banner Good Samaritan Medical Center, Department of Medicine, Phoenix, AZ |
| Anne-Michelle Ruha, MD | Banner Good Samaritan Medical Center. Department of Medical Toxicology, Phoenix, AZ Banner Good Samaritan Poison Control and Drug Information Center, Phoenix, AZ |
ABSTRACT
Latrodectus envenomations are common throughout the United States and the world. While many envenomations can result in catecholamine release with resultant hypertension and tachycardia, myocarditis is very rare. We describe a case of a 22-year-old male who sustained a Latrodectus envenomation complicated by cardiomyopathy.
INTRODUCTION
Widow spiders (genus Latrodectus) are very common, with more than 30 different species worldwide.1 In 2008, 2,524 suspected black widow envenomations were reported to United States Poison Control Centers,2 although this likely underestimates the true incidence of envenomation. Following envenomation, latrodectism, a syndrome characterized by severe muscular pain, abdominal pain and diaphoresis, is relatively common. More severe envenomations can result in agitation, nausea, hypertension, tachycardia, priapism and fasciculations.1,3 Myocarditis, as manifested by chest pain, an abnormal electrocardiogram (ECG) and echocardiogram, and elevated serum troponin has only rarely been described following black widow envenomation. Furthermore, this unique entity has only been described in Europe and Saudi Arabia and involved species that are not indigenous to the United States. 4–8 To our knowledge, severe myocarditis has not previously been described following envenomation from any of the four species of Latrodectus spiders (Latrodectus mactans, Latrodectus hesperus, Latrodectus geometricus, or Latrodectus variolus)commonly found in the United States.
CASE REPORT
A 22-year-old male was transferred to our institution for toxicological evaluation of a presumed black widow envenomation.
The patient’s past medical history was notable for fetal alcohol syndrome and a small congenital ventricular septal defect (VSD). He had no surgical history, except for placement of a chest tube after a traumatic injury five years prior to this presentation. He did not take any daily medications, and he denied using ethanol or illicit drugs but was a tobacco smoker of approximately one pack per day. Despite the history of fetal alcohol syndrome, he was highly functional and employed at a local store.
The patient was sitting outside on a bench at approximately 8 PM when he felt a sharp “poke” on his left thigh and saw a spider on his leg. Shortly thereafter, he developed lower back pain and abdominal pain. Tremors, diaphoresis and bilateral lower extremity paresthesias soon began. He presented to an emergency department (ED) where he was noted to have severe abdominal pain, prompting the clinicians to obtain a computed tomography (CT) of his abdomen and pelvis. His examination was notable for severe diaphoresis, periorbital edema, fasciculations and a target-like lesion over the left thigh. Throughout his 12-hour stay in the ED, he received three liters of normal saline, intravenous morphine, ketorolac, diphenhydramine and ranitidine. Because he was not improving, the decision was made to transfer the patient to our institution for toxicology evaluation.
During the transport, the patient received 100 mcg of intravenous fentanyl. Shortly thereafter, his oxygen saturation was noted to be in the mid 80s, prompting the administration of 2 mg of intravenous naloxone without improvement in the oxygenation. Supplemental oxygen was subsequently administered.
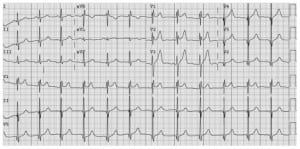
Upon arrival in the medical intensive care unit, the patient was noted to have a blood pressure of 157/94 mmHg and a heart rate of 103 beats per minute. His oxygen saturation was 89% on 4L of oxygen via nasal cannula. His exam was notable for diffuse muscle fasciculations without any muscular rigidity. He was diaphoretic and had distended neck veins with diminished lung sounds. A chest radiograph shortly after arrival revealed diffuse pulmonary edema, and an ECG revealed an incomplete right bundle branch block with ST elevations in the precordial leads (Figure 1). Laboratory studies were notable for a troponin of 1.37 ng/mL with a CK of 243 IU/L. An arterial blood gas, obtained while breathing 10L/min of oxygen via a simple face mask revealed a pH of 7.33, pCO2 of 42, and a pO2 of 74.6. The patient received 20 mg of intravenous furosemide, with significant diuresis and subsequent improvement in the hypoxia. The patient received one vial ofLatrodectus antivenom (“Antivenin [Latrodectus mactans], USP;” manufactured by Merck and Company) intravenously, which resulted in prompt normalization of the vital signs, and resolution of the diaphoresis, fasciculations, and pain.
Approximately two hours after arrival in the intensive care unit, and 15 hours after the envenomation, an echocardiogram revealed a left ventricular ejection fraction of 35–40% with mild to moderate tricuspid regurgitation and a right ventricular systolic pressure of 47 mmHg. No ballooning of the myocardium was noted on the echocardiogram. CT of the lungs revealed no evidence of pulmonary embolus. Repeat cardiac enzymes revealed decreasing troponin values. The patient was started on carvedilol 6.25 mg twice daily. A repeat echocardiogram 48 hours later revealed normalization of the left ventricular function and pressures. The patient was discharged home three days post envenomation.
DISCUSSION
We present a case of a severe black widow envenomation complicated by myocarditis, pulmonary edema, and a global, reversible, cardiomyopathy.
Following envenomation by the female widow spider, alpha-latrotoxin is the primary component of the venom, which is responsible for latrodectism.1 Alpha-latrotoxin is a 120 kilodalton protein, which binds to the presynaptic neuron and results in the exocytosis of multiple neurotransmitters, including norepinephrine, acetylcholine and glutamate from the presynaptic cell.9–11 This exocytosis occurs via both calcium-dependent and calcium-independent mechanisms.10
Alpha-latrotoxin can bind to the presynaptic receptor known as latrophin (also called Calcium Independent Receptor for alpha Latrotoxin [CIRL]). After binding occurs, the exact series of events is not known but may involve either induced mobilization of phospholipase C signaling with IP3intracellular calcium,9, 12 or it might involve a different and unknown G protein/second messenger system.10 Despite the exact intermediate steps not being known, it appears to result in the mobilization of intracellular calcium and subsequent exocytosis of neurotransmitters even in the absence of extracellular calcium.9–10, 13 In addition, at higher concentrations, alpha-latrotoxin can directly form cationic channels in the cell membrane, resulting in an influx of extracellular calcium, with subsequent further release and exocytosis of multiple neurotransmitters.10
The exact cause of the reversible cardiomyopathy in this individual is not known. We hypothesize that the catecholamine surge produced by alpha-latrotoxin resulted in a myocardial stun, causing a rapidly reversible myocarditis.
As with many patients with significant Latrodectus envenomations, our patient had hypertension, tachycardia, diffuse back, abdominal pain and profound diaphoresis.3 Despite not having the spider available for identification, given the constellation of symptoms and the temporal association between resolution of symptoms and the administration of antivenom, we believe this to be a black widow envenomation.
This individual did have a prior history of a congenital VSD. However, given the echocardiographic findings resolved 48 hours later, we do not believe that observed findings were the result of the VSD. Rather, we believe it was the result of the envenomation. It is not known if the prior congenital heart disease somehow predisposed him to myocarditis.
While myocarditis has been previously reported, albeit rarely, it has never been reported in North America. Given that envenomation from any member of the genus can likely produce a similar clinical picture, we do not have a clear explanation why myocarditis has not previously been described in North America, despite clearly having a large number of these spiders. Because the species of black widow differ in Europe and the United States, we believe this is the first case of myocarditis occurring following Latrodectus hesperus envenomation, the only Latrodectus species that inhabits the Southwest.
Footnotes
Supervising Section Editor: Jeffrey R. Suchard, MD
Submission history: Submitted December 11, 2009; Revision Received February 23, 2010; Accepted March 1, 2010
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Address for Correspondence: Michael Levine, MD. Department of Medical Toxicology. Banner Good Samaritan Medical Center. 925 East McDowell Road. 2nd Floor. Phoenix, AZ 85006
Email michael.levine@bannerhealth.com
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Vetter RS, Isbister GK. Medical Aspects of spider Bites. Annu Rev Entomol. 2008;53:409–29.[PubMed]
2. Bronstein AC, Spyker DA, Cantilena JR, et al. 2008 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 26th annual report. Clin Toxicol.2009;47:911–1084.
3. Clark RF, Wethern-Kestner S, Vance MV, et al. Clinical presentation and treatment of black widow spider envenomation: A review of 163 cases. Ann Emerg Med. 1992;21:782–87. [PubMed]
4. Erdur B, Turkcuer I, Bukiran A, et al. Uncommon cardiovascular manifestations after a Latrodectus bite. Am J Emerg Med. 2007;25:232–5. [PubMed]
5. Sari I, Zengin S, Davutoglu V, et al. Myocarditis after black widow spider envenomation. Am J Emerg Med. 2008;26:630e1–e3. [PubMed]
6. Puligano G, Del Sindaco D, Giovannini M, et al. Myocardial damage after spider bite (Latrodectus tredecimguttatus) in a 16-year-old patient. Giornale Italiano de Cardiologia. 1998;28:1149–53.discussion 1154–6.
7. Pneumatikos I, Galiatsou E, Goe D, et al. Acute fatal toxic myocarditis after black widow spider envenomation. Ann Emerg Med. 2003;41:158. [PubMed]
8. Bucur I, Obasi O. Spider bite envenomation in Al Baha region, Saudi Arabia. Ann Saudi Med.1999;19:15–9. [PubMed]
9. Ushkaryov YA, Volynski KE, Ashton AC. The multiple actions of black widow spider toxins and their selective use in neurosecretion studies. Toxicon. 2004;43:527–42. [PubMed]
10. Henkel AW, Sankaranarayanan S. Mechanisms of α latrotoxin action. Cell Tissue Res.1999;296:229–33. [PubMed]
11. Jelinke G. Widow spider envenomation (latrodectism): A worldwide problem. Wild Environ Med.1997;8:226–31.
12. Bittner MA, Holz RW. Latrotoxin stimulates secretion in permeabilized cells by regulating an intracellular Ca2+ -and ATP-dependent event: a role for protein kinase C. J Biol Chem.2000;275:25351–7. [PubMed]
13. Hlubek MD, stuenkel EL, Krasnoperov VK, et al. Calcium-independent receptor for α-latrotoxin and neurexin 1 α facilitate toxin-induced channel formation: evidence that channel formation results from tethering of toxin to membrane. Mol Pharmacol. 2000;57:519–28. [PubMed]


