| Author | Affiliation |
|---|---|
| Gus M. Garmel, MD | Stanford University School of Medicine/Kaiser Permanente, Santa Clara |
INTRODUCTION
Patients presenting to the emergency department (ED) with electrocardiograms (ECGs) indicating wide complex tachycardias (WCTs) are difficult to manage. Furthermore, these ECGs are often challenging to interpret.1,2 Patients typically have ongoing chest discomfort, with or without symptoms of dyspnea, lightheadedness, nausea, and diaphoresis. Accurate interpretation of ECGs demonstrating WCTs assists clinicians who must treat patients presenting with this condition. Despite step-wise approaches and numerous criteria suggested to interpret WCTs, physicians (including cardiologists) often fail to agree on a surface electrocardiographic diagnosis.3,4 Understanding the etiology of WCTs not only helps identify the cause of this condition, but also prevents its mismanagement, significantly reducing morbidity and mortality.5,6,7
This is the first of two manuscripts designed to remove the “complex” from wide complex tachycardia identification (part 1) and management (part 2). Information is provided to help clinicians interpret ECGs demonstrating WCTs, including descriptions of the electro- and pathophysiology behind their development. Several examples of WCTs are provided with detailed interpretations. Diagnostic criteria from current literature and their relative accuracy are presented. In part 2, the most recent 2005 American Heart Association (AHA) guidelines for the treatment of WCTs are discussed, and management strategies for various WCTs are described given their underlying etiologies.
Wide Complex Tachycardias (WCTs) are also known as Broad Complex or Wide QRS Complex Tachycardias. It is easiest to understand this nomenclature by considering these terms independently. Wide refers to a QRS complex duration (width) of greater than or equal to 0.12 seconds (120 msec), corresponding to three small boxes on the ECG paper. There are many reasons for QRS complexes to be widened (see Table 1). Any cause of a widened QRS complex can result in a sustained or nonsustained wide complex tachycardia if the rate is greater than 100 beats per minute. Etiologies of various WCTs are listed in Table 2, with schematic diagrams to better appreciate the conduction pathways and electrophysiology behind WCTs provided in Figure 1. The QRS Complex is the electrical stimulus on the ECG tracing as it passes from the AV node down the ventricular conduction system, terminating in the ventricular myocardial cells.8 This definition, however, proves to be rather limited, as the electrical stimulus that results in the QRS complex may travel in either the forward or backward direction (anterograde orretrograde) using various pathways as part of the conduction circuit (orthodromic orantidromic). The direction of and pathway used by the electrical impulse greatly affects the duration of the QRS complex, which may impact the heart rate achieved. Tachycardiais generally defined as any heart rate or pulse greater than 100 beats per minute, whether or not this is sustained.
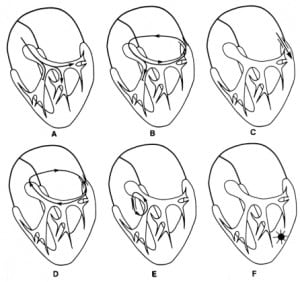
Illustrations showing different etiologies of WCTs using electrophysiological representations. (Wellens HJJ, Conover MB. The ECG in Emergency Decision Making. W.B. Saunders Co. Philadelphia, PA, 1992, p. 39). Used with permission.

Etiologies of a wide QRS Complex

Electrophysiologic and/or metabolic causes of WCT
Before reviewing diagnostic criteria recommended for the interpretation of ECGs demonstrating WCTs, clinicians must first recognize the importance of an individual’s presenting symptoms associated with this WCT. The initial assessment of the airway, breathing, and circulation (ABCs) is critical, as these determine the approach taken in treating the patient. However, these may or may not assist the clinician’s ability to identify the underlying condition responsible for the WCT. Oxygen and intravenous access should be started as soon as possible. Cardiac rhythm and blood pressure monitoring should be initiated immediately. A focused history and physical examination must be performed during this initial period, not only to gather additional information about this individual, but also to allow consideration for alternative diagnostic possibilities.
There are several aspects of the medical history that contribute to a clinician’s ability to identify the etiology behind a WCT. Individuals with previous myocardial infarction (MI) or known coronary artery disease (CAD) are approximately four times more likely to present with ventricular rather than supraventricular etiologies of their WCT. Stated another way, approximately 80% of all patients presenting with WCT will be diagnosed having VT as the cause.18 This figure may be higher than that seen in patients presenting to the ED, due to referral bias at specialty centers and the repeated citation of older literature.19 One small retrospective study found that histories of prior myocardial infarction (MI), congestive heart failure (CHF), and recent angina pectoris all had positive predictive values for VT greater than 95%, but sensitivities were low to moderate.20 None of these clinical characteristics was strongly predictive for SVT; the best was age less than or equal to 35 years (positive predictive value of 70%). In fact, this study concluded that for patients presenting with WCTs, there are no ECG findings or clinical variables highly suggestive of SVT with aberration. Previous Holter or electrophysiologically-proven VT, or medications used to treat VT may be helpful historically, as they make the diagnosis of VT more likely. However, neither is conclusive for VT as the etiology of the presenting WCT. Previous electrophysiologically-proven SVT does not exclude the diagnosis of VT, nor does it confirm the diagnosis of SVT, as individuals with a previous episode of SVT may present with an episode of VT. Positive responses to medications with prior episodes of “palpitations” or WCTs may not determine the etiology, as several medications work on blocking cardiac conduction both above and below the ventricle, and the etiology for the present rhythm disturbance may differ. Finally, previous adverse responses to cardiac medications may identify which medications to avoid in the management of an individual, regardless of the etiology of their presenting symptoms or ECG.
During a careful physical examination, identifying signs of atrio-ventricular (AV) dissociation strongly supports the and Electrophysiology diagnosis of VT. AV dissociationoccurs due to independent activity of the atria and ventricles, a characteristic occurrence in VT. Physical signs include irregular cannon A waves, varying intensity of the S1 heart sound, and beat-to-beat variability in systolic blood pressure (SBP). Irregular cannon A waves occur when the atria contract against AV valves that are closed because the ventricles are simultaneously contracting. These waves are the result of brisk blood backflow in the jugular veins. They do not occur with each cardiac cycle, and may be variable in quality because atrial contraction may occur when the AV valves are open or in differing stages of closure. This results in irregularity and varying quality of the cannon A wave intensity, when and if these waves occur. Varying intensity of the S1 heart sound is related to the timing of AV valve closure at the onset of ventricular systole. The beat-to-beat variability of the first heart sound’s loudness is due to the varying positions of the AV valves at the onset of ventricular systole. Beat-to-beat variability in SBP is due to the changing amount of blood in the ventricle during ventricular contraction, the result of varying diastolic filling times. On the ECG or rhythm strip tracings, the P waves and QRS complexes classically lack any relationship (i.e., there is no association between them), as demonstrated in Figure 2. This may be difficult to identify on ECGs demonstrating WCT, due in part to the tachycardia itself, as well as the morphology of the QRS complex. The absence of AV dissociation neither excludes the possibility that the WCT is ventricular in origin (VT), nor confirms that the WCT is supraventricular in origin. Vagal maneuvers, such as cautious carotid massage, gagging or coughing, and valsalva can slow the ventricular response in many tachycardias of supraventricular origin, allowing atrial activity to be more apparent. These maneuvers tend to be ineffective in tachycardias of ventricular origin.
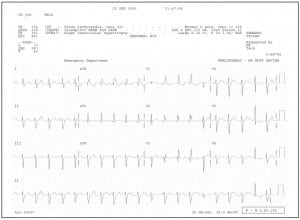
Lead II rhythm strip from a 12-lead ECG demonstrating AV dissociation in a patient with confirmed VT. Note that P waves march out independent of the QRS complexes, best seen in beats 2–10. Some of these P waves fall within or before the T wave, modifying its appearance. Also identified in lead II arefusion beats (QRS complexes #11, 15, and 20), and a narrow complex capture beat (next to last QRS complex #19).
The most important point to consider when evaluating and treating individuals presenting with WCTs is their hemodynamic status. An individual’s hemodynamic status does not help determine the etiology of the WCT. A survey of physicians published inJAMA in 1985 demonstrated this fact was commonly misunderstood.1 The determination of whether or not a WCT is unstable is based on the effect it has on end-organ perfusion. Neither the patient’s heart rate nor QRS width determines hemodynamic stability. Symptoms providing evidence of unstable WCTs include hypotension, pulmonary edema, confusion, and angina. Skin color, temperature, and moisture may also be considered as somewhat reliable predictors of end-organ perfusion. Many individuals with WCTs (or any tachycardias) will exhibit one or more of these symptoms to varying degrees, especially chest discomfort (angina). There is likely to be overlap between one or more of these symptoms as well, making bedside clinical judgment in the management of patients presenting with WCTs essential. An approach in which clinicians err on the side of considering someone hemodynamically “unstable” is most prudent. It is incorrect to consider that only VT can be unstable, or that SVTs of any etiology do not cause hemodynamic compromise.
Many patients with known cardiac histories (especially those with rhythm or conduction problems) take antidysrhythmic medications. These agents have various effects on cellular electrophysiology and are divided accordingly using the Vaughan-Williams international classification system. Most of these agents prolong the duration of various portions of the ECG (including the QRS complex), which may lead to the misinterpretation of WCTs. Furthermore, several of these agents are pro-arrhythmic because of their effects on conduction at the cellular level. Therefore, it is important to obtain a thorough medication history.
The age of an individual presenting with a WCT does not help determine its origin. Although WCTs in older individuals are more likely to be ventricular in origin (due to an increased incidence of previous MI or underlying CAD), the age of an individual is neither sensitive nor specific. Furthermore, an individual’s gender is not useful in determining the etiology of a WCT. Males are, however, more likely than females to have ventricular etiologies for their WCT, likely due to their increased incidence of prior structural cardiac disease and MI.
Neither the heart rate nor the width of the QRS complex in a single patient presenting with a WCT can definitively determine the origin of the rhythm disturbance. Many references state that a faster heart rate is more likely found in WCTs of supraventricular origin than in those of ventricular etiology. Ranges of heart rates that occur in SVT with aberrant conduction or VT are often given in a table, for example. However, presenting this information in this manner gives the illusion that there is a distinct heart rate above which VT does not occur. This is not true, as the range of heart rates found in SVTs and VTs overlap, depending on the underlying condition, medications, and physiological reserve of individuals experiencing these rhythms. Overall, the heart rate of WCTs due to aberrantly-conducted SVTs tend to be slightly faster than the rates due to VTs, but in a single individual at a given time, heart rate is not useful in determining the etiology of the WCT.
For patients with a permanent pacemaker, part of the physical examination diagnostics includes careful placement of a pacemaker magnet over a pacemaker generator. In patients presenting with a WCT, the magnet converts the pacemaker’s pulse generator from the synchronous (demand) to asynchronous (fixed or “magnet” rate) mode of response. This action may allow identification of atrial activity. If this WCT is pacemaker-mediated, the magnet is likely to terminate the abnormal rhythm.21
In addition to historical and physical examination clues, several diagnostic criteria exist using the rhythm strip and 12-lead ECG to help determine the etiology of a WCT (see Tables 3 – 7). In terms of the electrophysiology of the abnormal rhythm, a very regular rhythm tends to favor the diagnosis of SVT with aberrant conduction as the cause of the WCT. However, VT is generally considered as resulting in a regular wide complex rhythm. A markedly irregular rhythm favors atrial fibrillation (AFIB) with aberrant conduction, such as a preexisting Bundle Branch Block (BBB) or Wolff-Parkinson-White Syndrome (WPWS). Previous ECGs may provide essential information, such as prior rhythm patterns (normal sinus, AFIB, SVT, or VT) or previous QRS complex morphologies (BBB patterns, PVCs, fusion or capture beats during prior WCTs, electrical axis, evidence of WPWS, or structural heart disease). AV dissociation, although not commonly identified, and/or ventriculoatrial (VA) block, apparent in approximately 1% of ECGs demonstrating WCT, are very sensitive for VT. Fusion and capture beats are helpful in diagnosing VT, but are neither always present nor always reliable. Fusion beats are hybrid QRS complexes resulting from simultaneous supranodal (atrial) and infranodal (ventricular) activation of ventricular tissue. They are therefore intermediate in morphology and width from either a capture or ventricular beat. Capture beats are QRS complexes resulting in ventricular activation originating from supranodal tissue, using electrical conduction pathways above the ventricle. These are therefore narrow and are similar (or identical) to a “normal” QRS complex (see Figure 2). Precordial concordancemeans that the QRS “direction” on the ECG in all the precordial leads is consistent. Positive precordial QRS concordance may occur during VT or SVT using a left posterior accessory pathway for AV conduction. Therefore, positive precordial concordance does not discriminate between VT and SVT with aberrant conduction. Negative precordial concordance is nearly always VT, because antidromic circus movement tachycardia never has negative precordial concordance. According to Wellens, this is true since accessory pathways over which anterograde conduction leads to completely negative QRS complexes in all the precordial leads do not exist.22 However, a recent report of a 17-year-old male demonstrated that this “rule” is not perfect, as his underlying pectus excavatum and SVT with left BBB resulted in a WCT with negative precordial concordance due to SVT with aberrant conduction. This observation led the authors to conclude that “… no diagnostic technique is 100% correct and that there are always exceptions to the rule.”23,24 and Electrophysiology
Similar to the uncertainty of the history and physical examination in establishing the etiology of WCTs, ECG findings may create doubt as well. The duration (width) of the QRS complex is not very helpful in differentiating between supraventricular and ventricular etiologies of WCTs. A common misconception is that a QRS duration exceeding 0.14 second is present only if the rhythm is ventricular in origin (VT). However, in left BBB (V1-negative) WCTs, the QRS complex duration is often greater than 0.14 second even though the rhythm originates above the ventricles (i.e., supraventricular in origin). Even further misleading is that many antidysrhythmic agents cause QRS widening beyond 0.14 second, even if the rhythm originates above ventricular tissue.
Transesophageal atrial “pill” electrodes are used by electrophysiologists in an attempt to determine the etiology of underlying wide complex conduction abnormalities. These pills are “easily” swallowed by the patient, are secured adjacent to the atria, and may prove diagnostic.25 These electrodes record atrial activity (contraction) as large P wave spikes, which allows for easier identification of P waves and their relationship to QRS complexes. This may help identify AV dissociation or 1:1 VA conduction. A combination Esophageal Pill Electrode and Pacing Electrode may have a role in terminating certain tachydysrhythmias, such as atrial flutter, by overdrive pacing. The pill electrode has an unlikely role in the emergency department identification and management of WCTs due to its cost, availability, ease of use, safety, and lack of emergency physician experience using them. These pills are not indicated for patients experiencing hemodynamic compromise. The role of transesophageal electrocardiography in the diagnosis of WCT has received much attention (although little in the EM literature) as a safe, easy method for evaluating a WCTs’ mechanism.26,27,28 One case report describes the ease of bedside placement of a transesophageal lead in the ED in a patient with an uncertain WCT unresponsive to a number of medications. A diagnosis of VT was confirmed using this recording device, which demonstrated marked disparity between atrial and ventricular rates, with the ventricular rate exceeding that of the atrial rate.29
Research is ongoing using signal-averaging ECG technology, which may gain future acceptance assisting with the electrophysiological diagnosis of WCTs. There are reports of transcardiac lead systems, modified precordial lead use, and ice mapping of the AV node (reversible cooling of this tissue to test function prior to ablation) for identification of the etiologies of WCTs in electrophysiology laboratories.30,31,32 Presently, these technologies have limited roles in the ED.
Bedside ECG Diagnostic Criteria
Not all individuals presenting in a WCT are hemodynamically unstable. This is for the clinician to determine at the bedside. Once an individual has been deemed clinically stable, the ECG and rhythm strip should be reviewed closely for diagnostic clues to the etiology of the WCT. The “final” determination of the WCT should be considered an “academic exercise and not of the utmost importance even for a stable patient in the ED. It is perfectly acceptable for an emergency physician to provide a final interpretation of the abnormal ECG demonstrating a WCT as “wide complex tachycardia of uncertain (undetermined) etiology.” In fact, this may result in better patient care, as the focus of a patient presenting in a WCT should be on the patient’s hemodynamic status and management, not the “correct” interpretation of the ECG.
A WCT can occur in any individual presenting with tachycardia for any reason, such as fever or dehydation, if he or she has a pre-existing wide QRS complex. Other tachycardias that create or result in wide QRS complexes will by definition be WCTs. The best approach when considering the etiology of a WCT is to determine whether or not the rhythm and its associated tachycardia originate from above the ventricle (supraventricular), the ventricle itself, or is due to metabolic causes.
Several diagnostic criteria exist to assist clinician determination of the WCTs’ etiology. Much research has been published debating or, in some cases, modifying these criteria, in an effort to prove or discredit them as being the most accurate, reproducible, or easiest to use.33,34,35 Some researchers claim that the QRS axis helps determine the etiology of a WCT. ECG leads placed on the body create Einthoven’s triangle, which can be used to determine the overall electrical axis of a patient. The quadrant in which the QRS axis is found may provide additional support that the WCT pattern is due to VT (see Table 3).
The three most well-accepted criteria for the interpretation of WCTs based on the 12-lead ECG are the Wellens’ criteria (Table 4), Kindwall’s 4 electrophysiologic criteria for VT in LBBB (Table 5), and Brugada’s 4-step approach to regular tachycardias with wide QRS complexes (Table 6). Also included is a table using morphologic criteria of the ECG and its associated bundle branch pattern (positive and negative deflections) of the QRS (Table 7). The classification schemes based on QRS morphology are essentially the same; both are included because some individuals prefer classifying WCT ECGs as RBBB-like or LBBB-like, while others prefer to consider WCT ECGs according to V1(V2)-positive or negative classification schemes. The Brugada criteria are perhaps slightly favored by clinicians and/or cardiologists, although this may be because they are the most recent. All of these papers have generated further research and commentaries suggesting modification of these criteria, supported by examples demonstrating inappropriately classifiedWCTs when using these criteria. None of these approaches is favored, as each diagnostic set has inherent limitations resulting in ECG misidentifications.36,37 It is therefore prudent to be comfortable with more than one of these classification schemes, although many feel it is better to be very confident with just one approach.

Wellens’ Criteria (VT favored in the presence of)

Kindwall’s ECG criteria for VT in LBBB

Brugada’s 4-step Algorithm Approach40,41 This step-wise approach is performed as a series of questions. If the answer to any of these questions is “YES,” VT is identified and no further steps are made. If the criteria are not met for that step, the next question is asked
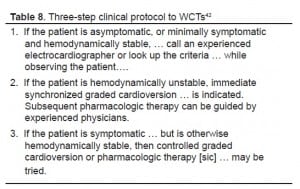
An interesting commentary published in 1989 following an article describing yet another set of criteria for identifying WCTs stated that “… knowing the specific criteria for diagnosis may not be that important.” The academic emergency physician who wrote this recommended instead that appropriate therapy should be dictated by a three-step protocol (Table 8).42 This simplified three-step protocol focuses on clinical criteria rather than electrophysiologic ones, which more closely resembles 2005 AHA guidelines. When in doubt, VT should be considered the default diagnosis in WCTs.43
UNDERSTANDING THE ECG IN WCTs
Understanding the electrophysiology behind ECGs with WCT assists clinicians with decision analysis and the explanation for abnormal conduction. Situations often exist during which a “normal” pathway of cardiac conduction does not occur. When this happens, the QRS complexes may appear different, having an increased duration or width. One such situation occurs during pre-excitation of the ventricle. Here, the normal electrical impulse originating from the atria reaches ventricular tissue earlier than expected by means of an accessory pathway. This impulse bypasses the AV node, which eliminates AV nodal delay and allows for earlier activation of the ventricle. The three most common accessory pathways identified are the Kent bundle (AV bypass tracts), responsible for the Wolff-Parkinson-White Syndrome (WPWS), the James’ responsible for the syndrome known as Lown-Ganong-Levine, and the Mahaim bundles (nodoventricular fibers). These accessory pathways each result in ventricular tissue receiving activation from a supraventricular source earlier than expected. This premature ventricular activation results in a shortened PR interval (the time from the beginning of atrial depolarization to the onset of ventricular depolarization). WPWS, which uses the Kent accessory bundle, is important because the QRS complex in individuals with this accessory pathway is widened to more than 0.10 seconds when this pathway is used.44,45This QRS complex widening occurs due to premature activation of the ventricle, not because of delayed activation (which occurs in BBB). WPWS has a slurred initial upstroke of the QRS known as a delta wave, the result of partial premature activation of ventricular tissue from the Kent accessory bundle combined with the majority of ventricular activation via the normal conduction pathway.46 This delta wave is therefore analogous to a fusion beat.
Reentry may be defined as an alternative pathway for cardiac conduction, with pre-excitation being one of its manifestations. These alternative pathways generally result in faster activation of ventricular tissue. They have varying periods of refractoriness, which is important when considering reentrant tachycardias. The Kent bundle, for example, is composed of faster conducting tissue than the AV node, but it has a longer refractory period. AV Nodal Reentry Tachycardias (AVNRTs) are tachycardias that use two distinct pathways within the AV node, having different refractory periods and conduction velocities. Circus Movement Tachycardias (CMTs), on the other hand, are reciprocating tachycardias that demonstrate either narrow or wide QRS complexes depending on the direction of reentry. An orthodromic reentry tachycardia means that AV conduction occurs in the normal direction (over the normal pathway). In antidromic CMTs, impulse conduction over the “expected” pathway occurs in the reverse direction. Here, an accessory pathway is used for anterograde (forward) conduction and the AV node or other accessory pathway is used in the retrograde (backward) direction as part of the conduction loop (see Figure 1). This results in wide QRS complexes, and therefore a WCT if the heart rate is greater than 100 beats per minute. Anterograde and retrograde are descriptive terms of convention; by themselves, they do not provide information about the configuration of the QRS complex nor the etiology of the WCT.
WPWS is the most common form of pre-excitation, using the Kent bundle as the accessory pathway for conduction. Left free wall pathways account for the majority of locations of the Kent bundle, although other locations for this accessory pathway exist in WPWS. Common ECG features of WPWS include short PR intervals (< 0.12 seconds), delta waves, and prolonged QRS intervals (> 0.10 seconds). Inverted T waves and increased precordial voltage may also be seen. Three types of WPWS are determined based on the location of the accessory pathway; however, they have little clinical significance other than identifying this syndrome. Type A WPWS has its accessory pathway in the inferoposterior portion of the LV, which results in a dominant R wave in lead V1 and Q wave in the inferior leads (the ECG configuration is therefore RBBB-like). Type B WPWS has its accessory pathway in the inferoposterior region of the RV. This results in an rS or QS pattern in lead V1 (the ECG configuration is therefore LBBB-like). Type C WPWS has its bundle in the posterolateral region of the LV, which results in negative or isoelectric delta waves in leads V5 or V6. The ECG exhibits small Q waves in leads I and aVL, commonly referred to as the pseudoinfarct pattern of WPWS.
WPWS is important because greater than 50% of individuals with these accessory pathways for AV conduction are predisposed to supraventricular tachydysrhythmias via reentry. Atrial fibrillation in individuals with WPWS may be extremely dangerous, as the Kent bundle may allow fast, chaotic atrial impulses to pass to ventricular tissue without delay at the AV node. Ventricular rates (identified as R-R intervals) may reach as high as 300 beats/minute in this situation. A clue to this is the presence of rapid, wide, and irregularly-spaced QRS complexes on the ECG.47,48,49 In this situation, clinicians must not administer agents that slow conduction through the AV node, as this makes conduction via the accessory pathway more likely. Therefore, antidysrhythmic agents that block AV nodal conduction or preferentially increase conduction through an accessory pathway in the situation of a WCT due to AFIB and WPWS can result in immediate hemodynamic collapse, ventricular fibrillation, or death.
CONCLUSION
WCTs are particularly challenging for clinicians to correctly identify. This is especially true given that most patients presenting to an emergency department with ECGs demonstrating WCTs are uncomfortable or have some hemodynamic distress. Correct interpretation of the WCT should not be the primary concern of emergency physicians; treating patients without doing harm takes precedence. Appropriate treatment approaches, including a detailed discussion of pharmacologic therapies, as well as additional (miscellaneous) causes of WCTs will be described in a forthcoming article.
Footnotes
Dr. Garmel is grateful to Bill H. Bosse, CBET (Northern California Regional Systems Administrator, Philips TraceMaster, Kaiser Foundation Hospitals) for formatting many of the ECGs included in this manuscript.
Submission history: Submitted August 18, 2007; Accepted November 26, 2007
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Address for correspondence: Gus M. Garmel, MD, FACEP, FAAEM Kaiser Permanente, C/O Emergency Department, 700 Lawrence Expressway, Santa Clara, CA 95051
Email: Gus.Garmel@Kp.org
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Morady F, Baerman JM, DiCarlo LA, et al. A prevalent misconception regarding wide complex tachycardias. JAMA. 1985;254:2790–2792. [PubMed]
2. Mason JW, Stinson EB, Winkle RA, Oyer PE. Mechanims of ventricular tachycardia: Wide, complex ignorance. Am Heart J. 1981;102:1083–1087. [PubMed]
3. Herbert ME, Votey SR, Morgan MT, et al. Failure to agree on the electrocardiographic diagnosis of ventricular tachycardia. Ann Emerg Med. 1996;27:35–38. [PubMed]
4. Isenhour JL, Craig S, Gibbs M, et al. Wide-complex tachycardia: Continued evaluation of the diagnostic criteria. Acad Emerg Med. 2000;7:769–773. [PubMed]
5. McGovern B, Garan H, Ruskin JN. Precipitation of cardiac arrest by verapamil in patients with Wolff-Parkinson-White syndrome. Ann Int Med. 1986;104:791–794.[PubMed]
6. Buxton AE, Marchlinski FE, Doherty JU, et al. Hazards of intravenous verapamil for sustained ventricular tachycardia. Am J Cardiol. 1957;59:1107–1110. [PubMed]
7. Stewart RB, Bardy GH, Greene HL. Wide complex tachycardia: Misdiagnosis and outcome after emergent therapy. Ann Int Med. 1986;104:766–771. [PubMed]
8. Dubin D. Rapid Interpretation of EKG’s. 3. Tampa, FL: C.O.V.E.R. Publishing Co.; 1970.
9. Garmel GM. Wide Complex Tachycardias. Hosp Phys: Emergency. Medicine Board Review manual. 1998;2:1–14.
10. Francis J, Hamzeh RK, Lantin-Hermoso MR. Lithium toxicity-induced wide complex tachycardia in a pediatric patient. J Peds. 2004;145:235–240.
11. Lange RA, Hillis LD. Cardiovascular complications of cocaine use. NEJM.2001;345:351–358. [PubMed]
12. Kerns W, Garvey L, Owens J. Cocaine-induced wide complex dysrhythmia. J Emerg Med. 1997;15:321–329. [PubMed]
13. Levis JT, Garmel GM. Cocaine-associated chest pain. Emerg Med Clin N Am.2005;23:1083–1103.
14. Clark RF, Vance MV. Massive diphenhydramine poisoning resulting in a wide-complex tachycardia: Successful treatment with sodium bicarbonate. Ann Emerg Med.1992;21:318–321. [PubMed]
15. Hollowell H, Mattu A, Perron AD, et al. Wide-complex tachycardia: beyond the traditional differential diagnosis of ventricular tachycardia vs. supraventricular tachycardia with aberrant conduction. Am J Emerg Med. 2005;23:876–889. [PubMed]
16. Kefalas S, Ezenkwele U. Wide-complex tachycardia as the presenting complaint in a case of malingering. J Emerg Med. 2006;30:159–161. [PubMed]
17. Knight BP, Pelosi F, Michaud GF, et al. Clinical consequences of electrocardiographic artifact mimicking ventricular tachycardia. NEJM. 1999;341:1270–1274. [PubMed]
18. Brady WJ, Skiles J. Wide QRS complex tachycardia: ECG differential diagnosis. Am J Emerg Med. 1999;17:376–381. [PubMed]
19. Akhtar M, Shenasa M, Jazayeri M, et al. Wide QRS complex tachycardia: Reappraisal of a common clinical problem. Ann Int Med. 1988;109:905–912. [PubMed]
20. Baerman JM, Morady F, DiCarlo LA, de Buitleir M. Differentiation of ventricular tachycardia from supraventricular tachycardia with aberration: Value of clinical history. Ann Emerg Med. 1987;16:40–43. [PubMed]
21. Barber CR, Garmel GM. Pacemaker-associated Tachycardia. Acad Emerg Med.1997;4:150–153. [PubMed]
22. Wellens HJJ, Conover MB. The ECG in Emergency Decision Making. Philadelphia, PA: WB Saunders Co.; 1992.
23. Volders PGA, Timmermans C, Rodriguez LZ, et al. Wide QRS complex tachycardia with negative precordial concordance: Always a ventricular origin? J Cardiovasc Electrophys. 2003;14:109–111.
24. Kappos KG, Andrikopoulos GK, Tzeis SE, et al. Wide-QRS-Complex tachycardia with a negative concordance pattern in the precordial leads: Are the ECG criteria always reliable? PACE. 2006;29:63–66. [PubMed]
25. Lopez JA, Lufschanowski R, Massumi A. Transesophageal electrocardiography and adenosine in the diagnosis of wide complex tachycardia. Texas Heart Inst Jour.1994;21:130–133.
26. Brembilla-Perrot B, Beurrier D, Houriez P, et al. Wide QRS complex tachycardia. Rapid method of prognostic evaluation. Internat J Card. 2004;97:83–88.
27. Koscove E. Diagnosis of Ventricular Tachycardia. Ann Emerg Med. 1996;28:244.[PubMed]
28. Jenkins J, Wu D, Arzebacher R. Computer diagnosis of supraventricular and ventricular arrhythmias: A new esophageal technique. Circ. 1979;60:977–987.
29. Haley JH, Reeder GS. Wide-complex tachycardia. Circ. 2000;102:e52.
30. Bella PD, Brugada P, Lemery R, et al. A Transcardiac lead system for identification and termination of supraventricular and ventricular tachycardia. Am J Card.1987;60:1043–1050. [PubMed]
31. Drew BJ, Scheinman MM. Value of electrocardiographic leads MCL1, and other selected leads in the diagnosis of wide QRS complex MCL6 tachycardia. JACC. 1991;18:1025–1033. [PubMed]
32. Gula LJ, Skanes A, Krahn AD, Klein GJ. Novel approach to diagnosis of a wide-complex tachycardia. J Cardiovasc Electrophys. 2004;15:466–469.
33. Gutierrez-Macias A, Sanz-Prieto JC, Aguirre-Herrero J, et al. Wide-complex tachycardia: Diagnostic Value of the Brugada algorithm in emergency medicine. Acad Emerg Med. 2001;8:300–301. [PubMed]
34. Pollack A, Falk RH. New criteria for diagnosis of regular, wide-complex tachycardias.Circ. 1992;85:1955–1956.
35. Dassen WRM, Karthaus VLJ, Talmon JL, et al. Evaluation of new self-learning techniques for the generation of criteria for differentiation of wide-QRS tachycardia in supraventricular tachycardia and ventricular tachycardia. Clin Cardiol. 1995;18:103–108. [PubMed]
36. Littmann L, McCall MM. Ventricular Tachycardia May Masquerade as Supraventicular Tachycardia in Patients with Preexisting Bundle-Branch Block. Ann Emerg Med. 1995;26:98–101. [PubMed]
37. Olshansky B. Ventricular tachycardia masquerading as supraventricular tachycardia: A wolf in sheep’s clothing. J Electrocard. 1988;21:377–384.
38. Wellens HJJ, Bar FWHM, Lie KI. The value of the electrocardiogram in the differential diagnosis of a tachycardia with a widened QRS complex. Am J Med. 1978;64:27–33.[PubMed]
39. Kindwall KE, Brown J, Josephson ME. Electrocardiographic criteria for ventricular tachycardia in wide complex left bundle branch block morphology tachycardias. Am J Cardiol. 1988;61:1279–1283. [PubMed]
40. Brugada P, Brugada J, Mont L, et al. A new approach to the differential diagnosis of a regular tachycardia with a wide QRS complex. Circ. 1991;83:1649–1659.
41. Antunes E, Brugada J, Steurer G, et al. The differential diagnosis of a regular tachycardia with a wide QRS complex on the 12-Lead ECG: Ventricular tachycardia, supraventricular tachycardia with aberrant intraventricular conduction, and supraventricular tachycardia with anterograde conduction over an accessory pathway.PACE. 1994;17:1515–1524. [PubMed]
42. Wrenn KD. Wide QRS-Complex tachycardia. Ann Int Med. 1989;110:412. [PubMed]
43. Griffith MJ, Garratt CJ, Camm AJ. Ventricular tachycardia as default diagnosis in broad complex tachycardia. Lancet. 1994;343:386–388. [PubMed]
44. Basson CT. A molecular basis for Wolff-Parkinson-White syndrome. NEJM.2001;344:1861–1864. [PubMed]
45. Lerman BB, Basson CT. High-risk patients with ventricular preexcitation – A pendulum in motion. NEJM. 2003;349:1787–1789. [PubMed]
46. Moore GP, Munter DW. Wolff-Parkinson-White syndrome: Illustrative case and brief review. J Emerg Med. 1989;7:47–54. [PubMed]
47. Nelson JA, Knowlton KU, Harrigan R, et al. Electrocardiographic manifestations: Wide complex tachycardia due to accessory pathway. J Emerg Med. 2003;24:295–301.[PubMed]
48. Panotopoulos P, Deshpande S, Akhtar M, et al. Wide QRS complex tachycardia in the presence of preexcitation: A diagnostic challenge. PACE. 1997;20:1716–1720. [PubMed]
49. Reddy CP, Sartini JC, Kuo CS. Paroxysmal ventricular tachycardia in Wolff-Parkinson-White syndrome: Case report and review of the literature. J Electrocardiol.1982;15:403–409. [PubMed]