| Author | Affiliation |
|---|---|
| Joshua Glick, BS | Penn State Hershey Medical Center, Penn State College of Medicine, Hershey, Pennsylvania |
| Erik Lehman, MS | Penn State Hershey College of Medicine, Department of Public Health Sciences, Hershey, Pennsylvania |
| Thomas Terndrup, MD | The Ohio State University, College of Medicine, Department of Emergency Medicine, Columbus, Ohio |
Introduction
Methods
Results
Discussion
Limitations
Conclusion
ABSTRACT
Introduction
Coordination of the tasks of performing chest compressions and defibrillation can lead to communication challenges that may prolong time spent off the chest. The purpose of this study was to determine whether defibrillation provided by the provider performing chest compressions led to a decrease in peri-shock pauses as compared to defibrillation administered by a second provider, in a simulated cardiac arrest scenario.
Methods
This was a randomized, controlled study measuring pauses in chest compressions for defibrillation in a simulated cardiac arrest model. We approached hospital providers with current CPR certification for participation between July, 2011 and October, 2011. Volunteers were randomized to control (facilitator-administered defibrillation) or experimental (compressor-administered defibrillation) groups. All participants completed one minute of chest compressions on a mannequin in a shockable rhythm prior to administration of defibrillation. We measured and compared pauses for defibrillation in both groups.
Results
Out of 200 total participants, we analyzed data from 197 defibrillations. Compressor-initiated defibrillation resulted in a significantly lower pre-shock hands-off time (0.57 s; 95% CI: 0.47–0.67) compared to facilitator-initiated defibrillation (1.49 s; 95% CI: 1.35–1.64). Furthermore, compressor-initiated defibrillation resulted in a significantly lower peri-shock hands-off time (2.77 s; 95% CI: 2.58–2.95) compared to facilitator-initiated defibrillation (4.25 s; 95% CI: 4.08–4.43).
Conclusion
Assigning the responsibility for shock delivery to the provider performing compressions encourages continuous compressions throughout the charging period and decreases total time spent off the chest. However, as this was a simulation-based study, clinical implementation is necessary to further evaluate these potential benefits.
INTRODUCTION
During cardiac arrest, significant pauses occur during resuscitation, particularly during defibrillation and endotracheal intubation.1,2 Results from several porcine and human studies suggest that these pauses are detrimental to survival.3–5 Furthermore, a higher chest compression fraction (CCF) has been shown to correlate with increased chances of survival for out-of-hospital arrest patients in both shockable and non-shockable rhythms.6–7 Moreover, longer pre-shock pauses in chest compressions have been associated with defibrillation failure,8 decrease in the likelihood of return of spontaneous circulation (ROSC),9 and a decrease in survival to hospital discharge.10 Associations between post-shock pauses and health outcomes have been mixed.
Current American Heart Association (AHA) and European Resuscitations Council (ERC) guidelines stress the importance of minimizing interruptions to chest compressions during cardiac arrest. Using manual over automatic defibrillation eliminates the need for lengthy computer analysis of pre-shock rhythm and minimizes the no-flow fraction.11,12 However, manual defibrillation has been associated with a higher frequency of inappropriate shocks and is rapidly falling out of clinical use over defibrillation with pads applied to the chest.13 Moreover, both the AHA and ERC currently recommend continued chest compressions while the defibrillator is being charged, an action that only recently has been considered to be safe for healthcare providers.14–16
Fear of accidental shock to a provider during defibrillation efforts often leads to an increase in the duration of peri-shock pauses and no-flow fraction. Traditionally, during an in-hospital cardiac arrest, one provider is usually responsible for performing chest compressions and a second provider is responsible for attaching the pads, charging the defibrillator, and delivering the shock. We propose a modified protocol in which a single provider is responsible for performing chest compressions and delivering the defibrillation shock. A second provider would still be responsible for attaching the pads and charging the defibrillator. Modifying which provider is responsible for the administration of a shock may decrease the duration of peri-shock pauses because of provider certainty about safe shock delivery and lower the risk of accidental defibrillation. We hypothesized that combining the responsibilities of shock delivery and chest-compression performance may lower no-flow periods and ultimately improve the probability of successful resuscitation.
METHODS
Design
This was a prospective, randomized controlled study measuring peri-defibrillation pauses of trained healthcare providers in a simulated cardiac arrest scenario. The institutional review board approved the research study via exemption.
Setting and Population
Participants were recruited in the local emergency department (ED) and from the Colleges of Medicine and Nursing. Prior to enrollment, all participants had completed a certified basic life support course in the previous 2 years and provided verification of such. All participants were 18 years of age or older.
Study facilitators included 8 medical students who all received 2 hours of training regarding the use of simulation equipment and study protocols. All facilitators received a standardized set of verbal instructions to provide use during each assessment and performed several practice sessions to ensure that differences in facilitation and data collection were minimized. Instructions were also provided for extracting data from the recording software.
Protocol
During enrollment periods in the ED, we asked potential study participants (technicians, nurses, physicians) to participate if time permitted. Arrangements were made for staff members to participate at a later time if requested. We recruited all medical and nursing students by e-mail for participation. Interested students were scheduled for screening and enrollment at the Clinical Simulation Center. Participants were randomized to either a control or study group using a permuted block list.
After obtaining verbal consent, participants were then informed that a simulated patient represented by a nearby mannequin (Laerdal Resusci Anne® Simulator, #150-0001, Wappingers Falls, NY) was experiencing “cardiac arrest with a known shockable rhythm.” The scenario information was provided in advance in order to remove the effects of variability in rhythm interpretation and focus on the measurement of differences in peri-shock pauses due exclusively to the defibrillation strategy. All subjects were asked to complete 1 minute of chest compressions on the simulation mannequin. Subjects were told when 30 and 45 seconds had elapsed and, at 50 seconds, that a defibrillator was being charged. After 1 minute of chest compressions, the facilitator notified the participant that a shock was ready.
All participants were currently trained in basic life support including a recent emphasis on reducing the duration of no-flow periods. Participants in the experimental group were instructed to clear by-standers, and after the facilitator confirmed that participants were clear, the participant administered defibrillation. These participants were instructed to continue chest compressions while clearing the patient. In the control group, the facilitator requested that compressions be continued during the charging period and then stopped in order to clear the patient prior to administering a shock. Defibrillation was performed by the facilitator immediately upon all participants being clear for safe defibrillation. All participants were instructed to resume compressions immediately after defibrillation. Each participant repeated the scenario 3 times, which allowed the experimental group to practice and become comfortable with the alternative technique. Accordingly, we used data from the third scenario for analysis.
Measurements
Following each simulated resuscitation, data regarding pre-shock and peri-shock times were measured and recorded for each participant (Laerdal PC SkillReporting System, #317000, Wappingers Falls, NY). We defined the pre-shock time period as the time from release of the last chest compression until administration of defibrillation. The peri-shock period was defined as the time from release of the last chest compression until the start of the first chest compression after defibrillation.
We collected and managed study data using Research Electronic Data Capture (REDCap) electronic data capture tools hosted at our facility. REDCap is a secure, web-based application designed to support data capture for research studies.
Statistical Methods
We computed descriptive statistics for all variables using means for continuous variables and percentages for categorical variables. After determining that the distributions of the outcome variables were approximately normal, we used a two-sample T-test to assess the statistical significance of the difference in mean pre-shock pause and in mean peri-shock pause between the control and study groups. All analyses were carried out using SAS Software version 9.2 (SAS Institute, Inc., Cary, NC).
RESULTS
During the period of July 2011 to October 2011, we recruited a total of 200 students and staff for participation in this study. Of these participants, complete and accurate data were available from 197 evaluations. Half of the participants (n=100) were enrolled in the control group and the remaining participants were enrolled in the study group (n=100). Demographic data for all study participants are shown in Table 1. The mean ages of the control and study groups were 29.4 ± 9.1 and 27.5 ± 7.0, respectively. The control group was 41% male, while the study group was 52% male. The majority of participants for both groups were medical students, but subjects also included nursing students, technicians, nurses, and physicians.
Table 1. Demographics of participants in study measuring pauses in chest compressions for defibrillation.
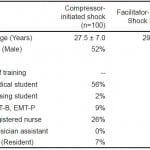 Table 2 highlights the differences in pre-shock and peri-shock pauses between the study group (compressor-administered defibrillation) and the control group (facilitator-administered defibrillation). The mean pre-shock pause time for the study group was significantly lower than the pause time of the control group (0.57 s versus 1.49 s, p<0.001). Furthermore, subject-initiated defibrillation resulted in a significantly lower peri-shock time compared to facilitator-initiated defibrillation (2.77 s versus 4.25 s, p<0.001).
Table 2 highlights the differences in pre-shock and peri-shock pauses between the study group (compressor-administered defibrillation) and the control group (facilitator-administered defibrillation). The mean pre-shock pause time for the study group was significantly lower than the pause time of the control group (0.57 s versus 1.49 s, p<0.001). Furthermore, subject-initiated defibrillation resulted in a significantly lower peri-shock time compared to facilitator-initiated defibrillation (2.77 s versus 4.25 s, p<0.001).
Table 2. Differences in mean pause time for defibrillation.
DISCUSSION
We sought to determine if no-flow time would be reduced by combining the roles of chest compression performance and administration of defibrillation. In a simulation model, we found that the revised protocol resulted in a statistically significant reduction in peri-shock pauses, but the overall reduction in no-flow time was small.
Several studies have demonstrated that lengthy pauses in chest compressions for procedures such as defibrillation or endotracheal intubation can have a negative impact on the probability of a successful resuscitation. In a study using porcine models, Yu et al.5 demonstrated that pauses in chest compressions as brief as 10 seconds prior to defibrillation can lengthen the time required to obtain ROSC and ultimately decrease the probability of successful resuscitation. Using observational human data, Edelson et al demonstrated that successful defibrillation was associated with each 5-second decrease in pre-shock pauses.8 In a second observational study, Sell et al.9 found that the likelihood of ROSC in human patients was associated with an optimal pre-shock pause of less than 3 seconds. These studies suggest that during cardiac arrest, longer periods without chest compressions prior to administering defibrillation can decrease the likelihood that resuscitation will be successful.
Our data demonstrated that combining the responsibilities of compression performance and defibrillation led to a significant decrease in pre-shock pauses. Given that prior research has shown that pauses greater than 3 seconds can have an impact on patient survival, the combination of roles used in our study may serve as one opportunity to decrease pre-shock pauses and possibly improve the likelihood of successful resuscitation.
Moreover, while automatic defibrillation requires a lengthy pre-shock interval for rhythm analysis and charging, the use of a manual defibrillator does not require the same pauses and allows compressions to be performed until just prior to administration of defibrillation.11,12 The most recent guidelines of both the AHA and the ERC reflect these findings and recommend that chest compressions be performed while the defibrillator is being charged.14,15 In a simulation study by Perkins et al.16 charging the defibrillator with concurrent administration of chest compressions was perceived as safe by participants and led to decreases in pre-shock pauses. Furthermore, a recent study by Lloyd et al.17 found that providers performing chest compressions during defibrillation were exposed to minimal and safe levels of leakage current. This further strengthens the suggestion that performing chest compression up to and during defibrillation may be a safe procedure that can further minimize the no-flow fraction.
Our study results suggest that the combination of compression administration and defibrillation facilitates continuous compressions during the charging period, thereby decreasing the pre-shock interval. During a resuscitation, it is likely that a provider will continue to administer chest compressions during the charging period if the risk for accidental defibrillation is minimized. A provider who is in control of both compression and defibrillation may feel more comfortable providing compressions while the defibrillator is being charged, knowing that the risk of accidental shock delivery is minimized.
LIMITATIONS
Our study has 3 important limitations. First, variability in the research protocol among facilitators is possible. The facilitator was involved with the process of defibrillation and thus may have had an effect on the pause times for the control group. All facilitators were instructed to follow a scripted procedure for pausing compressions, clearing the patient, and administering defibrillation. The scripted procedure was rehearsed several times during the training process and was available during the data collection period. Nonetheless, any variability among the facilitators may have led to discrepancies in the collected pause times.
Second, while our data demonstrated a statistically significant difference in pause times, whether these findings are clinically significant remains untested. This study was conducted in a controlled environment and only 2 providers (participant and facilitator) were present during each evaluation. In an actual hospital setting, having multiple providers is common and may complicate the direct communication that was available during the simulation. However, the clinical utility of having the chest compressor push the defibrillation activation button may be much greater when multiple providers and noisy communications, may contribute to longer no-flow periods during defibrillation. Additionally, during our simulation the defibrillator was always within reach of both the participant and the facilitator, and the pads were appropriately placed on the mannequin. During a live resuscitation, the defibrillator and pads are not always immediately available to the provider. Finally, additional aspects of complex resuscitations, such as multiple intubation attempts and central line placement, can further increase the no-flow fraction and are not accounted for in our simulation.
To determine whether the differences in pause periods are truly significant, the adaptations in provider responsibilities used in this study need to be implemented in a clinical setting and data regarding both no-flow periods and patient survival should be collected. Alternatively, a secondary study could be performed with more than 2 providers in each simulation team, which would more accurately simulate the conditions of a live resuscitation. This secondary study could also include multiple rounds of compressions and defibrillations in a longer ACLS scenario to more accurately incorporate additional factors such as provider fatigue, group communication, and hands-off time for rhythm checks or intubation.
CONCLUSION
In a simulated cardiac arrest, assigning the responsibility for shock delivery to the provider performing compressions encourages continuous compressions throughout the charging period and significantly decreases total time spent off the chest. This modification may also decrease the risk of accidental shock and improve patient outcomes. However, as this was a simulation-based study, clinical implementation is necessary to further evaluate these potential benefits.
Footnotes
Address for Correspondence: Joshua Glick, BS, Address. Email: glickjosh@gmail.com. 3 / 2014; 15:246 – 250
Submission history: Revision received April 15, 2013; Submitted July 4, 2013; Accepted September 16, 2013
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Abella BS, Alvarado JP, Myklebust H, et al. Quality of cardiopulmonary resuscitation during in-hospital cardiac arrest. JAMA. 2005; 293:305-310.
2. Wik L, Kramer-Johansen J, Myklebust H Quality of cardiopulmonary resuscitation during out-of-hospital cardiac arrest. JAMA. 2005; 293:299-304.
3. Yu T, Weil MH, Tang W, et al. Adverse outcomes of interrupted precordial compression during automated defibrillation. Circulation. 2002;106:368-372.
4. Steen S, Liao Q, Pierre L, et al. The critical importance of minimal delay between chest compressions and subsequent defibrillation: a haemodynamic explanation. Resuscitation. 2003;58:249-258.
5. Walcott GP, Melnick SB, Walker RG, et al. Effect of timing and duration of a single chest compression pause on short-term survival following prolonged ventricular fibrillation. Resuscitation. 2009;80:458-462.
6. Vaillancourt C, Everson-Stewart S, Christenson J The impact of increased chest compression fraction on return of spontaneous circulation for out-of-hospital cardiac arrest patients not in ventricular fibrillation. Resuscitation. 2011; 82:1501-1507.
7. Christenson J, Andrusiek D, Everson-Stewart S, et al. Chest compression fraction determines survival in patients with out-of-hospital ventricular fibrillation. Circulation. 2009; 120:1241-247.
8. Edelson DP, Abella BS, Kramer-Johansen J, et al. Effects of compression depth and pre-shock pauses predict defibrillation failure during cardiac arrest. Resuscitation. 2006; 71:137-145.
9. Sell RE, Sarno R, Lawrence B, et al. Minimizing pre- and post-defibrillation pauses increases the likelihood of return of spontaneous circulation (ROSC). Resuscitation. 2010; 81:822-825.
10. Cheskes S, Schmicker RH, Christenson J Perishock, et al. Pause: An Independent Predictor of Survival From Out-Of-Hospital Shockable Cardiac Arrest. Circulation. 2011; 124:58-66.
11. Pytte M, Pedersen TE, Ottem J, et al. Comparison of hands-off time during CPR with manual and semi-automatic defibrillation in a manikin model. Resuscitation. 2007; 73:131-136.
12. Berg RA, Hilwig RW, Kern KB, et al. Automated External Defibrillation for Prolonged Ventricular Fibrillation: Lethal Delays of Chest Compressions Before and After Countershocks. Ann Emerg Med. 2003; 42:458-466.
13. Kramer-Johansen J, Edelson D, Abella BS, et al. Pauses in chest compression and inappropriate shocks: A comparison of manual and semi-automatic defibrillation attempts. Resuscitation. 2007; 73:212-220.
14. 2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2005; 112:IV1-203.
15. Deakin CD, Nolan JP, Sunde K ERC, et al. Guidelines for Resuscitation 2010, Section 3: Electrical Therapies: Automated External Defibrillators, Defibrillation, Cardioversion and Pacing. Resuscitation. 2010; 81:1293-1304.
16. Perkins GD, Davies RP, Soar J, et al. The impact of manual defibrillation technique on no-flow time during simulated cardiopulmonary resuscitation. Resuscitation. 2007; 73:109-114.
17. Lloyd MS, Heeke B, Walter PF, et al. Rescuers in direct contact with patients during biphasic external hands-on defibrillation: An analysis of electrical current flow through defibrillation. Circulation. 2008; 117:2510-2514.