| Author | Affiliation |
|---|---|
| Risa S Moriarity, MD | University of Mississippi Medical Center, Department of Emergency Medicine, Jackson, Mississippi |
| Sylvia Dryer, MPH | University of Mississippi Medical Center, Department of Emergency Medicine, Jackson, Mississippi |
| William Replogle, PhD | University of Mississippi Medical Center, Department of Emergency Medicine, Jackson, Mississippi |
| Richard L Summers, MD | University of Mississippi Medical Center, Department of Emergency Medicine, Jackson, Mississippi |
ABSTRACT
Introduction:
The majority of patients seeking medical treatment for snakebites do not suffer from severe envenomation. However, no guidelines exist for ordering coagulation markers in patients with minimal or moderate envenomation, nor in those who do not receive antivenom. In this study, we sought to determine whether it was possible to limit the practice of ordering coagulation studies to those patients suffering severe envenomation, rattlesnake envenomation, or both.
Methods:
A retrospective chart review was performed on all cases of crotalid snakebite presenting to an adult emergency department (ED) from April 1998 to June 2006. Each chart was abstracted for patient’s age, gender, type of snake (if known), severity of envenomation at initial presentation, coagulation test results, whether antivenom was administered, and whether the patient was admitted.
Results:
Over an approximately 8-year period, 131 snakebite cases presented that met the inclusion criteria, of which 35 (26.7%) had some type of coagulation marker abnormality. Limiting coagulation testing to patients suffering severe envenomation or rattlesnake envenomation would have resulted in failure to identify 89% or 77%, respectively, of the 35 patients who were found to have at least 1 abnormal coagulation marker.
Conclusion:
Our study failed to identify a subset of patients that could be defined as low risk or for whom coagulation marker testing could be foregone. This study suggests that coagulation tests should be routinely performed on all patients presenting to the ED with complaints of envenomation by copperheads, moccasins, or rattlesnakes. Further clarification of when coagulation markers are indicated may require a prospective study that standardizes snake identification and the timing of coagulation marker testing.
INTRODUCTION
More than 2,800 venomous snakebites were reported to the American Association of Poison Control Centers in 2008.1 Venomous snakes in the Southeastern United States include rattlesnakes, copperheads, and water moccasins of the crotalid family, as well as coral snakes of the elapid family. A small number of bites by these poisonous snakes are termed dry, when little or no venom is actually injected and symptoms of envenomation do not develop. Envenomation is generally defined as occurrence of a snakebite plus evidence of tissue damage and can result in a spectrum of clinical symptoms and laboratory abnormalities from mild, local tissue injury to systemic illness, including hypotension, neuromuscular dysfunction, and coagulopathy.2 For a known envenomation, standard management includes advanced life support, if indicated, immobilization of the affected limb, local wound care, tetanus immunization booster, and analgesia. Patients are usually observed in the emergency department (ED) setting for 6 to 8 hours. Antivenom (CroFab by Protherics Inc, Brentwood, Tennessee) is typically given for progressive injury, with progression being defined as a worsening of local tissue injury, systemic manifestations, or coagulation abnormalities by laboratory testing.2
No clear guidelines exist for ordering coagulation markers in patients with minimal or moderate envenomation, nor in those who do not receive antivenom. Many ED physicians routinely order coagulation markers on all patients with snakebites, regardless of type of snake or severity of envenomation. The costs of platelet counts, prothrombin times (PT), activated partial thromboplastin times (aPTT), and fibrinogen concentrations are significant and contribute to the expense of the management of these patients. Further costs may also be incurred simply by keeping the patient in the ED longer than necessary. In this study, we sought to determine whether coagulation markers are indicated for all snakebite patients in our region or whether we could limit the practice to ordering these tests on only those patients suffering severe envenomation, rattlesnake envenomation, or both.
METHODS
A retrospective chart review was conducted for all cases of snakebite presenting to a university medical center adult ED from April 1998 to June 2006. Prior to chart review, 1 abstractor was trained by the principal investigator on the data collection process. The abstractor was not blinded to the study’s hypothesis. Inclusion criteria were age greater than 15 years, documented historical and clinical evidence of snakebite, and any of 4 coagulation markers recorded. Exclusion criteria were a known preexisting coagulopathy or hypercoagulable state, ED presentation delayed more than 6 hours, charts with insufficient data to determine the severity of envenomation, and charts with no coagulation markers recorded. Data was collected from an electronic medical record system. Data not included in the electronic record was reviewed in paper charts to gather remaining data variables. Case information used in our study included the ED physicians’ notes, nurses’ drug administration notes, and laboratory values.
Each chart was abstracted using a standardized data collection form for age, gender, type of snake, if known, severity of envenomation at initial presentation, coagulation test results (platelet count, PT, aPTT, and fibrinogen concentration), whether antivenom was administered, and whether the patient was admitted. In cases where the snake was not identified, it was recorded as unknown. Severity of envenomation at the time of presentation was taken directly from the physicians’ notes, if documented. If not explicitly recorded by the ED physician, physical examination and laboratory data were used to classify the envenomation as minimal, moderate, or severe using the severity scoring guidelines published by Gold et al in 2002.2 The severity scoring guidelines are detailed in Table 1. When a patient received antivenom at another hospital prior to transfer to the ED, these vials were included in the total number recorded. The hospital laboratory’s standard ranges were used to determine whether coagulation markers were normal or abnormal. In cases where a patient had serial coagulation markers documented, the most abnormal measurement for each coagulation marker was used.

Data were analyzed using SPSS version 18.0 (Chicago, Illinois). Chi-square tests were used to test for associations between nominal variables. Mann-Whitney U and Kruskal-Wallis H tests were used to test for differences in mean rank of the dependent variables when there were 2 and more than 2 levels, respectively, of the independent variable. Logistic regression was also used to test the relationship between various risk factors and the presence or absence of an abnormal coagulation marker. The criterion for statistical significance was P < 0.05.
RESULTS
Over the approximately 8-year period, 132 snakebite cases presenting to the ED met the inclusion criteria. There was only 1 patient bitten by a coral snake, and this patient was excluded from all subsequent analyses. The study sample of 131 included 87 (66.4%) men and 44 (33.6%) women. The mean age was 43.3 years (range 16–90). Forty-nine patients (37.4%) were bitten by copperheads, 29 (22.1%) by moccasins, 17 (13.0%) by rattlesnakes, and 36 patients (27.5%) could not identify the snake. There were 37 (28.2%) minimal, 86 (65.6%) moderate, and 8 (6.1%) severe envenomations. Seventy-two patients (55.0%) received antivenom, 57 (43.5%) did not receive antivenom, and we were unable to determine if 2 (1.5%) patients received antivenom. Among those patients administered antivenom, a median of 10 vials was used in the course of their treatment. Thirty-four patients (26.0%) were admitted to the hospital and the remainder discharged following ED observation and treatment. During routine laboratory testing, some type of coagulation marker abnormality was identified in 35 (26.7%) of the 131 snakebite patients. Seventeen (13.8%) had an abnormal PT, 17 (13.9%) had an abnormal aPTT, 8 (6.2%) had thrombocytopenia, and 5 (13.2%) had abnormal fibrinogen concentrations. The range of abnormalities is shown in Table 2. In the group of 35 patients with a coagulation marker abnormality, 89% were classified as having a mild or moderate envenomation, and 77% did not have a rattlesnake envenomation. Only 1 of the 131 patients in our study had documented bleeding in the ED. This patient suffered a severe rattlesnake envenomation and was noted to have hematemesis while in the ED.

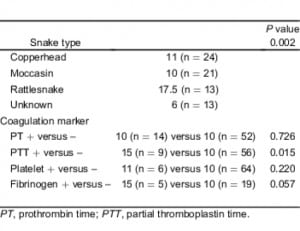
There were statistically significant associations between the identified type of snake and PT (P = 0.015), aPTT (P = 0.043), and fibrinogen (P = 0.028) abnormalities. Rattlesnake envenomation was associated with the greatest rate of coagulation abnormalities for each marker. Among patients envenomated by a rattlesnake, approximately 35%, 35%, and 40% had abnormal PT, aPTT, and fibrinogen concentrations, respectively. These percentages were more than double of those found for other snake types. There was also a significant association between the type of snake identified and the systemic symptoms experienced by the patient (P = 0.035). The percentage of rattlesnake envenomated patients with systemic symptomatology (35%) was twice the combined percentages experienced by patients envenomated by other snake types. There was no significant association found between the snake type and the frequency of observed thrombocytopenia (Table 3).

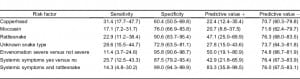
There was a significant association between the graded severity of envenomation and a laboratory finding of a PT abnormality (P = 0.021) for patients with moderate and severe envenomations being more likely to have a PT abnormality. There was also an association between the patient’s systemic symptomatology and abnormalities found on some of the coagulation makers (PT [P = 0.063), aPTT [P = 0.066], platelet [P = 0.005], and fibrinogen [P = 0.001]; Table 4). Sensitivity, specificity, and positive and negative values of each risk factor are shown in Table 5. The association between administration of antivenom and a finding of a PT abnormality was significant (P = 0.016), However, the association between administration of antivenom and a finding of a fibrinogen abnormality was not significant (P = 0.096; Table 4). Patients with abnormal fibrinogen concentrations tended to receive more vials of antivenom as compared to patients with normal concentrations, though this difference was not found to be statistically significant (P = 0.057; Table 6).
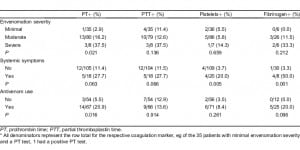
Median number of antivenom vials administered.
There was a significant association between the type of snake identified and a decision to administer antivenom (P = 0.018). A total of 76% of patients with a rattlesnake envenomation and 72% of patients with a moccasin envenomation received antivenom treatment. Only 49% of patients bitten by a copperhead and 41% of patients envenomated by an unknown snake type received antivenom (Table 3). Among patients who received antivenom, there was a significant difference in the number of vials administrated during the course of treatment when considering the specific snake types identified (P = 0.002) with rattlesnake victims receiving twice the number of vials as compared to patients bitten by other snake types. Patients with abnormal PTT tests also received more vials of antivenom (P = 0.015) than patients with other laboratory findings. We failed to find a significant difference in the number of vials administered and observed PT or platelet abnormalities (Table 6).
We performed an analysis using at least 1 abnormal coagulation marker as the outcome variable. Rattlesnake bite (+/−) was significantly associated with an abnormality (P = 0.04), and systemic symptoms were marginally associated with an abnormality (P = 0.068). Forty-seven percent of rattlesnake patients had a coagulation marker abnormality versus 23.7% of nonrattlesnake patients, relative risk was 1.99 (95% confidence interval [CI], 1.09–3.63). Severity and individual snakes (copperhead, moccasin, or unknown) failed to reach statistical significance. We then performed a binary logistic regression using at least 1 coagulation marker as the response variable. Severity (severe versus not severe), systemic symptoms (+/−), and rattlesnake bite (+/−) were used as predictors. The P value associated with severity was 0.974, and severity was dropped from the model. In the subsequent model, we used systemic symptoms and rattlesnake plus an interaction term as predictors. The interaction was found to be nonsignificant (P = 0.093). Main effects for both systemic symptoms and rattlesnake bites were significant (P = 0.044 and P = 0.035, respectively). Patients with systemic symptoms had a 13-fold (odds ratio [OR]) increase in the odds of an abnormal marker (OR = 13.33; 95% CI, 1.069–166.37). A rattlesnake bite was also associated with 13-fold increase in odds of an abnormal marker, (95% CI, 1.207–156.64). Finally, we performed a logistic regression with a dichotomous predictor representing those positive for both systemic symptoms and rattlesnake bite and those not positive for both. This dichotomy was a significant predictor (P = 0.013), and being positive for both systemic symptoms and rattlesnake bite was associated with increased odds of an abnormal marker of 15.8 (95% CI, 1.779–140.89). Finally, in this patient population, the positive and negative predictive values for this dichotomy were 83.3% and 76.0%, respectively (Table 5).
DISCUSSION
While it is common practice to order coagulation studies on patients with severe snakebite envenomations, the role of these tests for patients with mild or moderate envenomation is less certain. In this study, we attempted to determine whether coagulation markers are critical in the evaluation of all snakebite patients in our region or whether we could limit the practice to those patients suffering severe envenomation, rattlesnake envenomation, or both. The results of this study indicate that limiting such laboratory studies in this way could result in a failure to identify a large proportion of patients with abnormal coagulation markers. Restricting coagulation marker testing to patients suffering severe envenomation or rattlesnake envenomation would have resulted in our missing coagulation marker abnormalities in 89% or 77% patients, respectively. Restricting coagulation testing to patients with both a rattlesnake bite and systemic symptoms would have resulted in missing 86% of patients with coagulation marker abnormalities.
It is not surprising that patients bitten by rattlesnakes were more likely to have abnormal coagulation studies and greater systemic symptomatology as compared to patients bitten by other snakes. Also, it was not unexpected that patients with severe envenomations, and in which a clinical decision to use antivenom was made, were more likely to have coagulation marker abnormalities. Most clinicians who routinely treat snakebites are aware of the importance of performing these studies in these subsets of snakebite victims. However, in our experience, this practice has become a routine part of the evaluation and management of virtually all snakebite cases with limited objective evidence of necessity or benefit. A differentiation of a low-acuity patient subset in which this testing would not be required could potentially result in better resource utilization. The most interesting result of this study was the discovery that coagulation abnormalities were not confined to patients with severe envenomation, nor to patients with rattlesnake bites. Coagulation abnormalities were detected in 24% of patients bitten by copperheads and 15% of patients bitten by moccasins. Abnormalities were also detected in nearly one third of patients with moderate envenomation. Furthermore, 19% of patients who could not identify the snakes had some laboratory abnormality. These findings suggest that routine coagulation marker testing in nearly all ED patients with snakebites may be an important part of their clinical monitoring and management.
LIMITATIONS
There are several reasons that could explain our failure to identify a subset of patients that do not require coagulation marker testing. In general, however, the limitations of our study probably resulted in an underestimation of the prevalence of coagulation marker abnormalities in the study population. Difficulties involved in accurately identifying a snake type at the time of a bite event are self evident. Unless documented as unknown, we assumed that patients correctly identified the snakes that bit them. Ninety-five out of 131 patients in our study reported being bitten by venomous snakes. This may be partially accounted for by selection bias; many people bitten by nonvenomous snakes presumably identify them correctly and do not seek medical attention. However, the majority of snakes in our region are nonvenomous, and it is possible that some patients in our study incorrectly identified the snakes that bit them as venomous.
In order to stratify our patients by severity of envenomation, we used the severity scoring guidelines published by Gold et al in 2002 (Table 1). These guidelines rely in part on subjective measurements, such as degree of edema, erythema, and ecchymosis. In addition, they are designed only for patients with known envenomation.2 Nevertheless, we chose to use this system, as no completely objective tool for clinical stratification currently exists.
Envenomation is also a dynamic process. It is often difficult to ascertain whether a bite represents true envenomation on initial ED presentation, and estimations of dry bites vary widely.2,3 The amount of venom injected varies by age, condition, and species of the snake, size of the victim, and many other factors. The clinical management of snakebites typically requires a longitudinal monitoring of the patient’s response to the envenomation, which many also vary greatly according to the location of the bite and the inherent physiology of the affected individual. As snake venom is a complex mixture of enzymes, the clinical responses to envenomation are myriad.4 The venom of many snake species contains several components that can induce hemorrhage, including fibrinolytics, platelet aggregation inhibitors, and hemorrhagins. All crotalid snake venoms are theoretically capable of causing some degree of coagulopathy.5 Copperhead venom contains a protein C activator, water moccasins carry beta fibrinogenases, and timber rattlesnakes carry serine proteases.4 Case reports even exist of presumed nonvenomous snakes causing coagulopathy.6 This biologic complexity and the evolving nature of snakebite signs and symptoms may make it difficult to classify an evenomation as severe early in the course of clinical management. When considering all these factors, it is perhaps not surprising to find such a prevalence of laboratory abnormalities among the patients in the current study.
Additionally, not all patients in our study had all 4 coagulation markers (PT, aPTT, platelets, and fibrinogen concentration) drawn. Timing of patient blood draws also varied among our patients. In most cases, our patients had their coagulation markers drawn immediately after being placed in an ED examination room. However, the delay from the time of the bite to presentation in the ED varied. Asymptomatic coagulopathy may, therefore, have occurred in some patients following their initial blood draw and would not have been detected in our study. Several case reports and studies exist that document patients with delayed coagulopathy following envenomation.7,8,9
Patient charts were not abstracted for hemoglobin or hematocrit, so we may have failed to detect occult bleeding during ED observation. Only 1 patient in 131 had documented bleeding in the ED. Our study also did not address whether knowledge of abnormal coagulation markers changed management or impacted patient outcomes.
CONCLUSION
In this study, we sought to determine whether all patients presenting to an ED in our region should have coagulation markers routinely drawn as part of their management. We hypothesized that this practice could be limited to ordering the tests only for patients suffering severe envenomation, rattlesnake envenomation, or both. However, our study failed to identify a group of patients that could be defined as low risk or for whom coagulation marker testing could be foregone. Instead, this study suggests that coagulation tests should be routinely performed on all patients presenting to the ED with complaints of envenomation by copperheads, moccasins, or rattlesnakes. The study had a number of limitations, including snake identification, severity of envenomation stratification, and collection of coagulation markers. Further guidance for ED physicians might be provided by a prospective, multicenter trial, enrolling only patients for whom the snake could be positively identified and in which the same coagulation markers were drawn at a standardized time.
Footnotes
Supervising Section Editor: Brandon K. Wills, DO, MS
Submission history: Submitted February 15, 2011; Revision received May 25, 2011; Accepted June 13, 2011
Reprints available through open access at http://escholarship.org/uc/uciem_westjem
DOI: 10.5811/westjem.2011.6.6729
Address for Correspondence: Richard L. Summers, MD
University of Mississippi Medical Center, Department of Emergency Medicine, 2500 N State St, Jackson, MI 39216
E-mail: rsummers@umc.edu
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding, sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Bronstein AC, Spyker DA, Cantilena LRJ, et al. 2008 Annual report of the American Association of Poison Control Centers National Poison Data System (NPDS); 26th annual report. Clin Toxicol.2009;47:911–1084. [PubMed]
2. Gold BS, Dart RC, Barish RA. Bites of venomous snakes. N Engl J Med. 2002;347:347–356.[PubMed]
3. Litovitz T, Klein-Schwartz W, White S, et al. 1999 annual report of American Association of Poison Control Centers toxic exposure surveillance system. Am J Emerg Med. 2000;18:517–574. [PubMed]
4. Hutton R, Warrell D. Action of snake venom components on the hemostatic system. Blood Rev.1993;7:176–189. [PubMed]
5. Geoffrey K, Isbister GK. Procoagulant snake toxins: laboratory studies, diagnosis, and understanding snakebite coagulopathy. Semin Thromb Hemost. 2009;35:93–103. [PubMed]
6. Li Q, Huang G, Kinjoh J, et al. Hematological studies on DIC-like findings in patients with snakebite in south China. Toxicon. 2001;39:943–948. [PubMed]
7. Boyer L, Seifert S, Clark R, et al. Recurrent and persistent coagulopathy following pit viper envenomation. Arch Int Med. 1999;159:7. [PubMed]
8. Miller M, Dyer J, Olson K. Two cases of rattlesnake envenomation with delayed coagulopathy. Ann Emerg Med. 2002;39:348. [PubMed]
9. Bogdan G, Dart R, Falbo S, et al. Recurrent coagulopathy after antivenom treatment of crotalid snakebite. South Med J. 2000;93:562–566. [PubMed]


