| Author | Affiliation |
|---|---|
| Travis D Olives, MD, MPH, MEd | Hennepin County Medical Center, Minneapolis, Minnesota |
| Benjamin S Orozco, MD | Regions Hospital Toxicology Education and Clinical Service, St Paul, Minnesota |
| Samuel J Stellpflug, MD | Regions Hospital Toxicology Education and Clinical Service, St Paul, Minnesota |
INTRODUCTION
Mephedrone and MDPV are both β-ketophenethylamine derivatives of cathinone, a compound isolated from the East African plant Catha edulis (khat, qat). Mephedrone is commonly referred to as plant food, MCAT, 4-MMC, meow meow, meph, and drone; MDPV is commonly called MTV, MDPK, Magic, and Super Coke. Both are structurally similar to amphetamines, with mephedrone sharing close similarities with methamphetamine and MDPV with ecstasy (3,4-methylenedioxymethamphetamine; MDMA). Bath salts pose an increasing public health risk in the United States, with reports of toxicity and mortality increasing along with calls to poison centers throughout the United States. Packages labeled with innocuous monikers such as White Ice, Ivory Wave, Ocean Snow, Lunar Wave, and Vanilla Sky intentionally belie the dangerous substances within, which are by no means intended to replace legitimate bath products. The white or tan crystalline powder commonly is administered by nasal insufflation or oral ingestion; however, rectal suppository and less commonly, intramuscular or intravenous injection, are also reported.1,2
A movement to ban these substances is growing in the United States, following similar actions in Europe.3 Although successfully outlawed in some locales, this movement has not eliminated the public health hazards posed by mephedrone or MDPV. Emergency physicians (EP) should thus be knowledgeable in the epidemiology of bath salt abuse, the clinical toxidrome with which bath salt toxicity presents, and appropriate treatment strategies to reduce morbidity and mortality in patients presenting with bath salt toxicity.
CLINICAL EFFECTS
Based on studies of similar compounds, mephedrone and MDPV may possess intrinsic stimulant properties owing to their effects on plasma membrane dopamine, norepinephrine, and serotonin transporters, resulting in both reuptake inhibition and direct agonist activity.1,4,5 The exact action of mephedrone and MDPV remains somewhat theoretical, as previously tested compounds have demonstrated varying effects despite their structural similarities. Norepinephrine and dopamine reuptake inhibition are likely prominent in MDPV and mephedrone, resulting in a sympathomimetic toxidrome similar to that of more familiar illicit substances with which most providers are more familiar, including cocaine, methamphetamine, and ecstasy.6
User reports describe a euphoric high lasting between 2 to 4 hours with prominent letdown effects lasting several hours afterward. Reported doses range from 5 to 10 mg for the more lipophilic MDPV (although 1 patient reported taking 2 g over an unclear time course) and 100 to 500 mg for mephedrone.7–10 Redosing of both is common. Euphoria, empathic mood, sexual stimulation, subjectively greater mental focus, and increased energy are reported in the highs of both substances.1,7,8 In a recent survey study of past mephedrone users, the 1,506 participants revealed that ecstacy compared most similarly. Significant complications have also been documented, including seizure activity, severe agitation, myocarditis, and chest pain, as well as compulsive dosing to sustain effect.8,11–13 Case fatalities resulting from bath salt consumption, as well as consumption of mephedrone and MDPV from other sources, have also been published in the literature.11,14–16
Some of the reported cases can shed light on the specific clinical manifestations.
A 36-year-old male in the Netherlands became acutely agitated and enraged after ingesting mephedrone along with cocaine, and subsequently lost consciousness and died despite resuscitation efforts.16 A 29-year-old male found unresponsive at a nightclub died of cerebral edema and brainstem herniation. Qualitative toxicologic blood screening revealed mephedrone, and no other substance, in his blood.11 Serum sodium was noted to be 125 mmol/L, later suggested by laboratory data to have resulted from water intoxication. The first synthetic cathinone-related death in the United States, described in the scientific literature, involved a 22-year-old male who was found unresponsive and subsequently died at the receiving hospital. Blood and urine tested positive for mephedrone, heroin metabolites, codeine, and doxylamine.14 One case of mephedrone-related myocarditis has also been reported in the literature.13 In this instance, a 19-year-old male presented with crushing chest pain after ingesting mephedrone sold as “not-for-human-consumption” plant food. Electrocardiographic changes with greater than 3 mm ST-segment elevation in the anterolateral leads, and high T2 signal at the lateral left ventricle on cardiac magnetic resonance imaging, confirmed the diagnosis of myocarditis. One additional case of documented 2-mm ST depression in a patient exposed to MDPV is reported, although this case did not result in death and resolved with only sublingual nitrates.10 MDPV was implicated by history, though never analytically confirmed, to be the cause of the death of a 24-year-old man reported by the lay press.17 A recent series of cases of analytically confirmed mephedrone toxicity verified the sympathomimetic toxidrome that accompanies its use and provided insight into the spectrum of care and disposition undertaken for acute mephedrone toxicity. Among these cases were 4 emergency-department (ED) and short-stay discharges, 2 ward admissions, and 1 intensive care unit admission/subsequent death, reported in 7 cases.11
SCOPE OF THE PROBLEM
Rising use patterns and cases of self-mutilation and suicide have garnered increasing media attention.18 A recent survey of young adult “clubbers” in England revealed that 33.6% of respondents had used mephedrone in the past month, nearing the last-month use of cocaine, MDMA, and ketamine. More than 40% of respondents reported ever having used mephedrone.19 While reports of deaths due to mephedrone and MDPV consumption litter popular media outlets, several have also been reported in the scientific literature.20–22 In a case series of 4 fatalities in Scotland in which mephedrone was detected in postmortem femoral blood samples, 2 of the deaths were officially attributed to mephedrone toxicity, 1 was attributed to an abdominal stab wound, and another was not described. In 2 of the 4 cases, β-keto amphetamines were not suspected but found on gas chromatography-mass spectrometry; multiple coingestants were identified in 3 of the cases.23 As with other sympathomimetic drugs of abuse, the morbidity and mortality associated with bath salts stem both from direct physiologic toxicity and from behaviors spurred by the drugs’ effects.
Incident usage of cathinone-derived sympathomimetic bath salts continues to increase in the United States. Data are limited, largely because unique surveillance coding of bath salt calls to poison centers was initiated only in mid 2010 (Bailey, personal communication, April 25, 2011). Nationally, 302 calls regarding bath salts were made to poison centers in 2010; by October 3, a total of 5,226 calls to poison centers had already been made in 2011 (Bailey, personal communication, October 3, 2011).24,25 These data represent all calls made to US poison centers during the described time periods, and certainly represent a small slice of all bath salt use and morbidity in the United States. By contrast, poison center calls referencing synthetic cannabinoids have not seen the same increase in volume. This is a comparison that may prove instructive for EPs: the designer synthetic marijuana movement is of similar scope, likely familiar to most EPs, and it seems to be on the decline, while bath salt use seems to be on the rise (see Figure).26
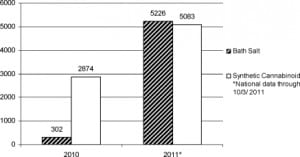
On September 7, 2011 the US Drug Enforcement Agency (DEA) announced that it will exercise its emergency scheduling authority and temporarily control MDPV and mephedrone (along with methylone, a similar compound), as schedule I substances for a minimum of 12 months.27 Senator Charles Schumer (D, New York), who had introduced legislation in February 2011 that urged defining both as schedule I controlled substances, now reports that he will pursue permanent scheduling.28–30 The DEA considers both MDPV and mephedrone analogs of methcathinone, a schedule I substance, and thus covered by the Federal Analogue Act, but only if intended for human consumption. At the time of the DEA’s announcement, at least 33 states had independently taken measures to control the substances specifically in bath salts.27–35
Some have suggested that scheduling these new “legal highs” has little effect on actual control of the substance, and that the shift from “legal” to illegal may result in increased risks for users owing to the possibility of adulterated manufacturing and reliance on street dealers.36,37 The results of previous bans on mephedrone suggest a dim end to the battle to reduce its use through legal actions. One instructive example of this is the ban on mephedrone enacted in the United Kingdom on April 16, 2010.38 A study of national poison center data in the year leading up to the ban revealed roughly 1,800 contacts with mephedrone-poisoned patients, incrementally increasing month by month.39 A survey completed 3 months after banning the substance, including 150 previous mephedrone users, demonstrated that two thirds of users continued using the drug despite its illegality.36
SUGGESTED EVALUATION AND MANAGEMENT
Specific recommendations for ED evaluation and management of isolated bath salts exposures, specifically for mephedrone or MDPV, would be difficult in most cases presenting to the ED, and interventions typically undertaken for more common sympathomimetic toxicities remain the first line of therapy. A specific antidote does not exist, and few laboratories have the capacity to screen serum or urine for specific bath salts; to our knowledge, none on a timeline useful in the acute care setting. Features typical of the bath salt toxidrome include, but are not limited to, altered mentation and sensorium, agitation, tachycardia, hypertension, and hyperthermia, with other symptoms possible as well. Such a presentation is not only common but also consistent with a wide range of disease states, both toxicologic and nontoxicologic. Even among patients with unequivocally toxicologic etiologies to explain these symptoms, bath salts are but one in a multitude of potential intoxicants and diagnoses, including serotonin syndrome, neuroleptic malignant syndrome, anticholinergic and sympathomimetic toxidromes, drug withdrawal syndrome, and exposure to older hallucinogens (ie, lysergic acid diethylamine, phencyclidine) or to a newer tryptamine or phenethylamine hallucinogen. At the moment, it remains advisable to keep designer β-ketophenethylamine cathinone derivatives such as mephedrone and MDPV on the differential diagnosis for patients presenting with the symptoms listed above or with toxidromes largely consistent with sympathomimetic toxicity.
Clinical experience in the treatment of bath salt toxicity is limited, and stratifying risk for certain outcomes that draw concern with other sympathomimetics or with new independent concerning outcomes is difficult. The risk for acute coronary syndrome, rhabdomyolysis, and serotonin syndrome, for example, remains unclear at this time, as does the subsequent need for laboratory testing and appropriate monitoring parameters. The case reports discussed above document important outcomes including death, myocarditis, agitated delirium, and hyponatremia, all of which merit considerable care in the management of documented or suspected bath salt toxicity. It thus seems prudent to include in the ED workup several key monitoring and therapeutic interventions. Peripheral intravenous access and cardiac monitoring are essential starting points, as is obtaining full vital signs at the outset of the visit including temperature, and repeating those vital signs during the ED stay. We recommend, at minimum, vital checks every 30 minutes until stable. Electrocardiograms and chest radiographs should be obtained for all patients presenting with tachycardia, chest pain, or shortness of breath. The specific role of cardiac markers in the evaluation of these patients has not been elucidated; however, given previously documented mephedrone cardiac toxicity13 and well-known propensity of amphetamines to cause direct cardiac damage by way of vasospasm and ischemia, it is prudent to approach the treatment of patients with acute bath salt toxicity and chest pain with at least the same level of caution as nonintoxicated patients with cardiac chest pain. A basic metabolic panel should be drawn for all patients to seek out hyponatremia and metabolic acidosis. The risk of rhabdomyolysis is uncertain, but in the setting of persistent agitation, obtaining a baseline creatine kinase would be reasonable. A complete blood count is unlikely to aid in the workup of bath salt toxicity, although in the setting of altered mental status, agitation, and hyperthermia of unclear etiology, it may be improper to ignore.
Agitation can be controlled with benzodiazepines as first-line therapy. Other supportive measures, including fluid management and temperature control, may play significant roles in individual cases. More advanced fluid management techniques may be required for cases complicated by hyponatremia or rhabdomyolysis. Seizures, should they occur, would be expected to respond to medications acting through γ-aminobutyric acid promotion, such as benzodiazepines, barbiturates, and propofol. Airway compromise, extreme sedation needs, seizures evolving to status epilepticus, and uncontrolled agitation all suggest the need for advanced airway management.
A controversial portion of the workup for any patient whose primary problem is exposure to a drug or poison is the “tox screen.” It should be noted that, in general, for emergency-department decision making for the toxicologic patient, this screening test is unhelpful.40 This notwithstanding, there is increasing precedent for detection of both mephedrone and MDPV. The first isolated mephedrone-related case report stated that the substance was not identified by routine toxicologic analysis, but subsequent reports had success with more advanced testing.2 Identification of mephedrone from samples seized in police raids has been recently described.41 Both mephedrone and MDPV have recently been identified in urine samples by gas chromatography-mass spectrometry, and mephedrone can be identified by liquid chromatography-mass spectrometry.42–45 MDPV has also been identified by nuclear magnetic resonance spectroscopic analysis.46 At least 1 commercial laboratory currently offers diagnostic testing on urine for both via liquid chromatography-tandem mass spectrometry with a reporting limit of 1.0 ng/mL.47 Despite these advances, a urine or blood toxicologic assessment negative for amphetamines in the face of convincing history and physical examination should not dissuade the astute EP from undertaking care appropriate for a bath salt ingestion. As with many emerging substances of abuse, little data are available to describe the sensitivity of currently used immunoassays in broad terms, and at this time it is likely that the clinical assessment of the patient who has ingested bath salts will be more sensitive for the diagnosis than a toxicologic screen.
CONCLUSION
There is a limited but building body of literature consisting largely of case reports, case series, surveys, media releases, and poison center data regarding mephedrone and MDPV toxicity. Chemical structure, case reports, popular media, user forums, and the sparse provider data that exist all support the notion that both compounds act to create a sympathomimetic toxidrome akin to that of cocaine and certain amphetamines. Proposed evaluation and management arise from experience and case reports only, but are likely congruous with standard proposed management of more well-known drugs such as cocaine, methamphetamine, and MDMA. Epidemiologic data are suggestive of a growing disease burden stemming from markedly increasing popularity of these so-called legal highs. Even in areas in which they have been banned, the problem of their acute toxicity persists and should be recognized by the well-prepared EP.
Footnotes
Supervising Section Editor: Jeffrey R. Suchard, MD
Submission history: Submitted April 28, 2011; Revision received June 21, 2011; Accepted June 28, 2011
Reprints available through open access at http://escholarship.org/uc/uciem_westjem
DOI: 10.5811/westjem.2011.6.6782
Address for Correspondence: Travis D. Olives, MD, MPH, MEd
Hennepin County Medical Center, 701 Park Ave, Mailcode 825, Minneapolis, MN 55415
E-mail: travis.olives@gmail.com
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding, sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Schifano F, Albanese A, Fergus S, et al. Mephedrone (4-methylmethcathinone; ‘meow meow’): chemical, pharmacological and clinical issues. Psychopharmacology (Berl) 2011;214:593–602.[PubMed]
2. Wood DM, Davies S, Puchnarewicz M, et al. Recreational use of mephedrone (4-methylmethcathinone, 4-MMC) with associated sympathomimetic toxicity. J Med Toxicol.2010;6:327–330. [PubMed]
3. Drugnet Europe. Mephedrone ban across the EU: news from the European Monitoring Centre for Drugs and Drug Addiction, January–March 2011. European Monitoring Centre for Drugs and Drug Addiction Web site. Available at:http://www.emcdda.europa.eu/publications/drugnet/online/2011/73/article2. Accessed April 25, 2011.
4. Cozzi NV, Sievert MK, Shulgin AT, et al. Inhibition of plasma membrane monoamine transporters by beta-ketoamphetamines. Eur J Pharmacol. 1999;381:63–69. [PubMed]
5. Nagai F, Nonaka R, Satoh Hisashi Kamimura K. The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain. Eur J Pharmacol. 2007;559:132–137.[PubMed]
6. James D, Adams RD, Spears R. et al. Clinical characteristics of mephedrone toxicity reported to the UK National Poisons Information Service. Emerg Med J. 2011;28:686–689. [PMC free article][PubMed]
7. Erowid. MDPV effects. Available at:http://www.erowid.org/chemicals/mdpv/mdpv_effects.shtml. Accessed February 19, 2011.
8. Erowid. 4-Methylmethcathinone (mephedrone, 4-MMC) the basics. Available at:http://www.erowid.org/chemicals/4_methylmethcathinone/4_methylmethcathinone_basics.shtml. Accessed February 19, 2011.
9. Carhart-Harris RL, King LA, Nutt DJ. A web-based survey on mephedrone. Drug Alcohol Depend.2011;118:19–22. [PubMed]
10. Durham M. Ivory Wave: the next mephedrone. Emerg Med J. [published online ahead of print March 15, 2011]
11. Wood DM, Davies S, Greene SL, et al. Case series of individuals with analytically confirmed acute mephedrone toxicity. Clin Toxicol (Phila) 2010;48:924–927. [PubMed]
12. Karila L, Reynaud M. GHB and synthetic cathinones: clinical effects and potential consequences.Drug Test Anal. 2011;3:552–559. [PubMed]
13. Nicholson PJ, Quinn MJ, Dodd JD. Headshop heartache: acute mephedrone ‘meow’ myocarditis.Heart. 2010;96:2051–2052. [PubMed]
14. Dickson AJ, Vorce SP, Levine B, Past MR. Multiple-drug toxicity caused by the coadministration of 4-methylmethcathinone (mephedrone) and heroin. J Anal Toxicol. 2010;34:162–168. [PubMed]
15. Gussow L. Ivory Wave” identified as MDPV. The Poison Review Web site. Available at:http://www.thepoisonreview.com/2010/08/20/ivory-wave/. Published 2010. Accessed February 16, 2011.
16. Lusthof KJ, Oosting R, Maes A, et al. A case of extreme agitation and death after the use of mephedrone in The Netherlands. Forensic Sci Int. 2011;206:e93–e95. [PubMed]
17. Jones S, Power M. Ivory Wave drug implicated in death of a 24 year-old-man. Guardian Web site.Available at: http://www.guardian.co.uk/society/2010/aug/17/ivory-wave-drug-alleged-death. Published 2010. Accessed April 5, 2011.
18. Warren T. Snorting bath salts pushed a St. Tammany man to suicide. The Times-Picayune Web site. Available at:http://www.nola.com/crime/index.ssf/2011/01/snorting_bath_salts_pushed_st.html. Published January 16, 2011. Accessed February 19, 2011.
19. Dick D, Torrance C. Mixmag drug survey. Mixmag. 2010;225:44–53.
20. Leicestershire. Death fall leads former drug user to warn of Ivory Wave. Northcliffe Media Limited Web site. Available at: http://www.thisisleicestershire.co.uk/news/Death-fall-leads-man-warn-legal-high/article-2545070-detail/article.html. Accessed February 16, 2011.
21. Craig T. Galveston dad wants “bath salts” banned after son’s death. KHOU-TV Inc Web site. Available at: http://www.khou.com/news/texas-news/Texas-could-consider-banning-Bath-Salts-114880629.html. Accessed February 16, 2011.
22. Maddux S. “Bath salts” result in near death. WSBT-TV Web site. Available at:http://articles.wsbt.com/2011-02-14/mephedrone-and-methylenedioxypyrovalerone_28535939. Accessed February 16, 2011.
23. Torrance H, Cooper G. The detection of mephedrone (4-methylmethcathinone) in 4 fatalities in Scotland. Forensic Sci Int. 2010;202:e62–e63. [PubMed]
24. Wehrman J. U.S. poison centers raise alarm about toxic substance marketed as bath salts; states begin taking action. American Association of Poison Control Centers Web site; Available at:http://www.aapcc.org/dnn/Portals/0/prrel/april20bathsalts.pdf. Accessed February 15, 2011.
25. Falkowski C. Drug abuse trends in Minneapolis/St. Paul, Minnesota. Minnesota Department of Human Services Web site. Available at:http://www.dhs.state.mn.us/main/groups/disabilities/documents/pub/dhs16_157630.pdf. Accessed February 15, 2011.
26. Wehrman J. Fake marijuana spurs more than 4,500 calls to U.S. Poison Centers Web site.American Association of Poison Control Centers Web site; Available at:www.aapcc.org/dnn/Portals/0/prrel/revisedk2releaseapril20.pdf. Accessed April 25, 2011.
27. United States Drug Enforcement Administration. DEA moves to emergency control synthetic stimulants [press release] Available at: http://www.justice.gov/dea/pubs/pressrel/pr090711.html. Accessed September 28, 2011.
28. Schumer CE. Schumer calls for ban of deadly synthetic drug MDPV, marketed as ‘bath salts’ but used by youth for meth-like high; drug, recently put on market, has led to several deaths and violent behavior by users throughout the country. press release]. Senator Charles E. Schumer Web site. Available at: http://schumer.senate.gov/new_website/record.cfm?id=330723&. Accessed February 15, 2011.
29. Braiker B, Moisse K. Bath salts: Sen. Charles Schumer looks to impose nationwide ban. ABC News Web site. Available at: http://abcnews.go.com/Health/Wellness/bath-salts-sen-charles-schumer-impose-nationwide-ban/story?id=12865604. Accessed February 15, 2011.
30. Division of Administration, State of Louisiana. Added controlled dangerous substances.Available at: www.doa.louisiana.gov/osr/reg/1101/1101.doc. Accessed February 15, 2011.
31. Ferran L. DEA announces emergency ban on ‘Bath Salts’. ABC News Web site. Available at:http://abcnews.go.com/Blotter/bath-salts-dea-announces-emergency-ban/story?id=14467134. Accessed September 28, 2011.
32. North Dakota Legislative Branch. North Dakota article 61-13: controlled substances schedules.Available at:http://www.legis.nd.gov/information/rules/docs/pdf/2010/pharbd042410changes.pdf. Accessed February 15, 2011.
33. Florida Department of Law Enforcement. Florida statute section 893.035(7)(a) (2011) Available at: http://www.fdle.state.fl.us/Content/getdoc/ffc3fe6e-ede9-4895-8263-2d50df7977fb/Bath-Salts.aspx. Accessed February 16, 2011.
34. CNN. Bath salts’ under close scrutiny after state bans. Available at:http://www.cnn.com/2011/CRIME/02/09/bath.salt.crackdown/index.html. Accessed February 16, 2011.
35. The Register Guard. Synthetic ‘bath salts’ outlawed in Oregon. Available at:http://www.registerguard.com/web/newslocalnews/26098889-41/synthetic-chemicals-oregon-bath-salts.html.csp. Accessed April 11, 2011.
36. Winstock A, Mitcheson L, Marsden J. Mephedrone: still available and twice the price. Lancet.2010;376:1537. [PubMed]
37. McElrath K, O’Niell C. Experiences with mephedrone pre- and post-legislative controls: perceptions of safety and sources of supply. Int J Drug Policy. 2011;22:120–127. [PubMed]
38. Sare J. Medicine and the media: how the media helped ban mephedrone. BMJ. 2011;342:472–473.
39. James D, Adams RD, Spears R, et al. Clinical characteristics of mephedrone toxicity, reported to the UK National Poisons Information Service. Emerg Med J. 2011;28:686–689. [PMC free article][PubMed]
40. Tenenbein M. Do you really need that emergency drug screen? Clin Toxicol. 2009;47:286–291.[PubMed]
41. Frison G, Gregio M, Zamengo L, et al. Gas chromatography/mass spectrometry determination of mephedrone in drug seizures after derivitization with 2,2,2-trichloroethyl chloroformate. Rapid Commun Mass Spectrom. 2011;25:387–390. [PubMed]
42. Meyer MR, Wilhelm J, Peters FT, et al. Beta-keto amphetamines: studies on the metabolism of the designer drug mephedrone and toxicological detection of mephedrone, butylone, and methylone in urine using gas chromatography-mass spectrometry. Anal Bioanal Chem. 2010;397:1225–1233.[PubMed]
43. Ojanperä IA, Heikman PK, Rasanen IJ. Urine analysis of 3,4-methylenedioxypyrovalerone in opioid-dependent patients by gas-chromatography-mass spectrometry. Ther Drug Monit.2011;33:257–263. [PubMed]
44. Strano-Rossi S, et al. Toxicological determination and in vitro metabolism of the designer drug methylenedioxypyrovalerone by gas chromatography/mass spectrometry and liquid chromatography/quadrupole time-of-flight mass spectrometry. Rapid Commun Mass Spectrom.2010;24:2706–2714. [PubMed]
45. Sorensen L. Determination of cathinones and related ephedrines in forensic whole-blood samples by liquid-chromatography–electrospray tandem mass spectrometry. J Chromatogr B.2011;879:727–737. [PubMed]
46. Westphal F, Junge T, Klein B, et al. Spectroscopic characterization of 3,4-methylenedioxypyrrolidinobutyrophenone: a new designer drug with a-pyrrolidinophenone structure. Forensic Sci Int. 2011;209:126–132. [PubMed]
47. MedTox. Mephedrone and MDPV. Available at:http://www.medtox.com/Resources/Images/6159.pdf. Accessed February 23, 2011.


