| Author | Affiliation |
|---|---|
| David Bordo, MD | Emergency Medicine Residency Program, Resurrection Medical Center, Chicago, IL |
| Shu B. Chan, MD, MS | Emergency Medicine Residency Program, Resurrection Medical Center, Chicago, IL |
| Peter Shin, MD | Emergency Medicine Residency Program, Resurrection Medical Center, Chicago, IL |
ABSTRACT
Introduction:
With regard to sedative agents used in procedural sedation and analgesia (PSA), such as etomidate, the focus has been on variables usually related to side effect profile and the success rates of various procedures, with both variables specifically taking place during the patients’ stay in the emergency department (ED). There have been no extensive data on the functional status of patients after they leave the ED following PSA.
Methods:
Prospective questionnaire evaluating functional status among consecutive adult patients discharged from the ED after undergoing etomidate PSA for orthopedic procedures.
Results:
The study sample consisted of 26 cases using only etomidate for closed orthopedic reductions. The mean age was 50.1 years (SD: 20.5), mean weight 86.3 kg (SD: 17.2), and 61.5% were males. The average dose of etomidate given was 0.14 mg/kg with 26.9% requiring a second dose of 0.11 mg/kg. The average dose of analgesic given was 0.11mg/kg in morphine equianalgesic units. The median time between procedural sedation and return to normal sleep was 36 hours, while return to operating a motor vehicle or return to work was 72 hours. Overall, 80% to 100% of respondents felt that any temporary dysfunction was secondary to the orthopedic problems and not to the procedural sedation.
Conclusion:
In this small follow-up study, adult patients undergoing PSA with etomidate for orthopedic closed reduction attribute post-discharge functional disability to the injury sustained and not to the PSA itself.
INTRODUCTION
Procedural Sedation and Analgesia (PSA), as noted from the 2005 American College of Emergency Physicians clinical policy,1 is defined as “…a technique of administering sedatives or dissociative agents with or without analgesics to induce a state that allows the patient to tolerate unpleasant procedures while maintaining cardio-respiratory function” (p.178).1 Although emergency department (ED) physicians use PSA for the repair of complex pediatric lacerations and electrical cardioversions, PSA is also commonly used for reducing fractures and dislocations.2
Many drugs are available for procedural sedation. Etomidate has been used as an anesthetic agent for over a quarter century.2–4 It is a non-barbiturate hypnotic that induces sedation through g-aminobutyric acid (GABA) receptors in the central nervous system (CNS).5 Its rapid onset, short duration of action, clinically insignificant hemodynamic alterations and minimal side effects have accorded it prominence as an adjunct to rapid sequence intubation in emergency medicine. Etomidate’s potential adverse effects include nausea/vomiting, potential loss of the protective airway reflexes, myoclonus, adrenal suppression, and hypotension. Electrolyte imbalances and oliguria may occur with prolonged use.5,6
Previous research regarding sedative agents focused on variables usually related to the side effect profile of the medication and the success rates of the procedures involved. In addition, some research focused on more qualitative variables. These variables, however, are limited to questions regarding patient satisfaction and appropriate recovery time until discharge.2,7 Quantitative studies on the functional status of patients after discharge from the ED following procedural sedation needs investigation. This study examined the effects on the recovery and return to daily activities of adult patients who underwent procedural sedation for orthopedic reductions in the ED.
METHODS
This is a prospective questionnaire evaluating functional status among consecutive adult patients discharged from the ED after undergoing etomidate PSA for orthopedic procedures. The study used a 10-item telephone questionnaire format. One to two weeks after discharge, investigators completed the formal questionnaire. Consecutive patients were identified from the ED log for all patients undergoing procedural sedation from September 2004 to June 2005. The study was performed at an urban community hospital that serves 37,000 annual patient ED visits.
Study Protocol
This prospective study initially examined medical records for specific demographic data about the patient to assure inclusion parameters (use of etomidate, ED patients older than 18 years of age and procedures involving orthopedic reduction with or without use of analgesics). Exclusion criteria included pregnant patients, non-English speaking patients, history of organic brain disease (not alert, and oriented to person, place, or time), and procedures conducted with analgesics but without the use of sedatives.
Measurements
Abstracted data included age, gender, weight, arrival and discharge times, time elapsed from sedation to discharge, diagnosis, names and doses of all medications related to the procedure, and complications. Anticipated complications reviewed included oxygen de-saturation (<94 %), blood pressure <90/60 mm Hg, blood pressure >140/90 mm Hg, respiratory rate <10 breaths per minute, respiratory rate > 20 breaths per minute, respiratory assistance, respiratory failure requiring intubation, mental status abnormality, vomiting, and rhythm changes. Investigators also recorded major medical conditions, allergies, use of tobacco, type of work, and exercise habits. Following acceptance by the Institutional Review Board, all data, including responses to the questionnaire, were recorded on preformatted data sheets, coded, and entered into an electronic database. Figure 1 shows the data collection tool.
Data Analysis
The Statistical Package for Social Sciences (SPSS) for Windows Version 11.5 (Chicago, IL, 2002) was used to calculate descriptive statistics.
RESULTS
Thirty patients received procedural sedation for closed orthopedic reductions during the study period. Two patients were excluded secondary to dementia. The remaining 28 patients were successfully contacted and agreed to participate in the study. However, one patient received both etomidate and diazepam for sedation and another patient received both etomidate and midazolam. These two cases were removed from analysis.
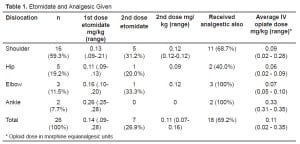
The mean age was 50.1 years (SD: 20.5), the mean weight was 86.3 kg (SD: 17.2) and 61.5% of the sample were males. The majority of patients were employed with only eight (30.8%) retired. An associated medical condition was noted in 10/26 (38.5%) patients and five of those had either hypertension or heart disease. Table 1 provides the type of orthopedic reduction and the average dose of etomidate used. A second dose of etomidate was required in 26.9% of cases.
Table 1 also provides the median dose of associated analgesic drugs used during procedural sedation. An intravenous analgesic along with etomidate was given in 18 (69.2%) patients. Morphine was given solely in nine (34.6%) cases, while six (23.1%) were given fentanyl only, and three (11.5%) received both.
Our study found no patients that experienced oxygen de-saturation, emesis, rhythm changes, or mental status abnormalities; however, four (15.4%) patients had slightly decreased or increased respiratory rates. None required intervention. Overall, 14 (53.8%) patients had alterations of systolic blood pressure, but only one patient required intervention. This patient received enalapril for elevated blood pressure. On the follow-up survey all 26 reported that the procedural sedative was extremely effective for amnesia to the events. All 26 also were willing to undergo the same sedation again. All 26 experienced nausea following ED discharge, but only one patient reported nausea for more than 12 hours.
Table 2 and Table 3 show the median time for return to normal activities and function. It is seen that in 80% to 100% of cases, patients felt that their temporary disability was secondary to their orthopedic issue rather than related to their procedural sedation.
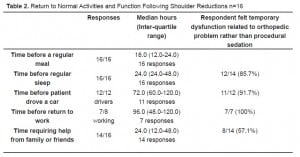
Return to Normal Activities and Function Following Shoulder Reductions n=16
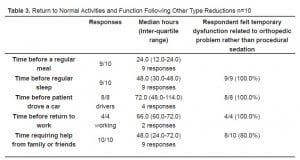
Return to Normal Activities and Function Following Other Type Reductions n=10
DISCUSSION
This study examined the effects of procedural sedation on ED-discharged adult patients and their recovery and return to daily activities. Etomidate was the primary sedative used for orthopedic reductions. Overall, patients receiving etomidate for PSA in the ED were satisfied and PSA was well tolerated. Of the 26 patients in our study, only one patient required any intervention for an adverse response. These results are consistent with previously published reports.2,4,5,8,9
The median time of functional disability for the patients in our study was one to three days. However, over 80% of the respondents felt that their disability, i.e., returning to normal sleep, operating motor vehicles, and returning to work, was not related to PSA. Almost all of the patients required family/friend assistance for a median of 1.5 days, and 57.1% of the patients felt that this was directly a function of PSA. These findings directly affect how emergency medicine physicians can educate their patients prior to discharge following PSA. Certainly, the medical condition that necessitated PSA will limit their activities for 1–3 days, but they should also be aware that they may need extra assistance from family and friends.
LIMITATIONS
A major limitation to this study is the small sample size. However, given the emerging popularity of etomidate PSA among emergency physicians and that much of PSA in the ED is performed for orthopedic reductions,2,4,9 these results are clinically relevant for many practicing emergency physicians. Finally, this study relied on accurate patient perceptions and recall. Some patients may have had trouble truly delineating the cause of the limitations, in other words, the medication involved in the procedure, or the medical condition necessitating the procedure.
CONCLUSION
Etomidate for orthopedic reductions in the ED is well tolerated with very high patient satisfaction and low adverse events; however, patients will have some temporary disability following discharge. Adult patients will likely have some functional disability for 36 to 72 hours. The vast majority of patients related their temporary functional disability to their underlying orthopedic problem and not to the procedural sedation itself.
Footnotes
Supervising Section Editor: Brandon Wills, DO, MS
Submission history: Submitted November 6, 2007; Revision Received March 18, 2008;Accepted March 20, 2008.
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Address for correspondence: Shu B. Chan, MD, MS, Resurrection Medical Center, Emergency Medicine Residency Program, 7435, West Talcott Avenue, Chicago, IL 60631
Email: schan@reshealthcare.org
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Godwin SA, Caro DA, Wolf SJ, Jagoda AS, Charles R, Marett BE, et al. Clinical policy: procedural sedation and analgesia in the emergency department. Ann Emerg Med.2005;45:177–196. [PubMed]
2. Vinson DR, Bradbury DR. Etomidate for procedural sedation in emergency medicine.Ann Emerg Med. 2002;39:592–598. [PubMed]
3. Doenicke A. Etomidate, a new intravenous hypnotic. Acta Anaesthesiol Belg.1974;25:307–315. [PubMed]
4. Ruth WJ, Burton JH, Bock AJ. Intravenous etomidate for procedural sedation in emergency department patients. Acad Emerg Med. 2001;8:13–18. [PubMed]
5. Keim SM, Erstad BL, Sakles JC, Davis V. Etomidate for procedural sedation in the emergency department. Pharmacotherapy. 2002;22:586–592. [PubMed]
6. Katzung BG. Basic and Clinical Pharmacology. 7. Stamford, CT: Appleton and Lange; 1998.
7. Newman DH, Azer MM, Pitetti RD, Singh S. When is a patient safe for discharge after procedural sedation? The timing of adverse effect events in 1367 pediatric procedural sedations. Ann Emerg Med. 2003;42:627–635. [PubMed]
8. Burton JH, Bock AJ, Strout TD, Marcolini EG. Etomidate and midazolam for reduction of anterior shoulder dislocation: a randomized, controlled trial. Ann Emerg Med.2002;40:496–504. [PubMed]
9. Dursteler BB, Wightman JM. Etomidate-facilitated hip reduction in the emergency department. Am J Emerg Med. 2000;18:204–208. [PubMed]