| Author | Affiliation |
|---|---|
| Erika Esteve, MD | Hospital del Mar, Department of Internal Medicine, Barcelona, Spain |
| August Supervía, MD, PhD | Hospital del Mar, Department of Emergency Medicine, Barcelona, Spain |
| Oriol Pallàs, MD | Hospital del Mar, Department of Emergency Medicine, Barcelona, Spain |
| María T. Martínez, MD | Hospital del Mar, Department of Emergency Medicine, Barcelona, Spain |
| María M. Montero, MD | Hospital del Mar, Department of Internal Medicine, Barcelona, Spain |
| Francisco del Baño, MD | Hospital del Mar, Department of Emergency Medicine, Barcelona, Spain |
INTRODUCTION
Tuberculosis was a major public health problem, given the estimated one billion people infected between 2000 and 2002.1 A decrease in the incidence of tuberculosis was observed in the last decade, coinciding with improvements in social and healthcare conditions and availability of more effective antituberculous therapies. However, in the last two or three years, this decreasing trend has been interrupted by the presence of two emerging factors, the influx of immigrants and infection by the human immunodeficiency virus (HIV).1 In the United States, this increase in the incidence of tuberculosis in recent years has been attributed, in part, to an increase in the diagnosis of this disease among immigrants from countries with a high prevalence of tuberculosis.2 The case of an immigrant patient presenting with a septic respiratory clinical picture in which the final diagnosis was miliary tuberculosis and HIV coinfection is reported.
CASE REPORT
A 31-year-man from Guinea, who had moved to Barcelona three years before consultation, was admitted to the emergency department because of a wasting syndrome, characterized by asthenia, anorexia and weight loss. These symptoms had been insidiously developed over the past three months. The patient complained of cough, mucopurulent expectoration, nausea and vomiting and was unresponsive to antibiotic treatment during the last week. Family history and personal history were unrevealing. He was an active smoker. Physical examination showed fever (38°C), a blood pressure of 75/35 mm Hg, a heart rate of 110 beats per minute and a respiratory rate of 32 breaths per minute with fatigue. Oxygen saturation breathing room air was 92%. Results of laboratory tests included serum creatinine level of 1.4 mg/dL, total bilirubin 2.1 mg/dL, aspartate aminotransferase 232 IU/L, C-reactive protein 20.8 mg/dL (normal range 0–0.8 mg/dL), white blood cell count 2060/μL (neutrophils 1860/μL, lymphocytes 160/μL), platelets 64000/μL and international normalized ratio 1.23. Baseline arterial blood gas analysis included PaO2 61 mm Hg, PaCO2 26 mm Hg and pH 7.46. The chest radiograph revealed a micronodular interstitial pattern affecting both hemithoraces (Figure 1).
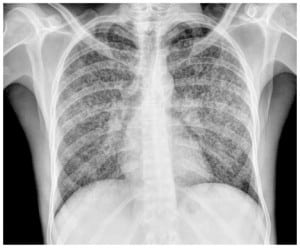
A tentative diagnosis of septic shock secondary to an infectious respiratory focus was established because of a lack of improvement after treatment with fluids. The patient received empirical antibiotic treatment with levofloxacin 500mg two times a day and trimethoprim sulfamethozaxole 160/800mg three times a day. A human immunodeficiency virus (HIV) test and Ziehl-Neelsen staining of the sputum smear were requested. The Ziehl-Neelsen test was positive (result was available at 12 hours of admission), and a diagnosis of miliary tuberculosis with pulmonary involvement was made. Quadruple antituberculous treatment (isoniazid, rifampin, pyrazinamide and ethambutol) was started. The clinical condition of the patient worsened, and he required admission to the intensive care unit (ICU). Continuous infusion of norepinephrine and invasive mechanical ventilation were required. One week after starting antituberculous treatment, the patient’s clinical condition improved and he was discharged from the ICU.
HIV testing was positive (C3 category), the CD4 cell count was 44/mm3 and the viral load 3,095,299 copies/mL. The sputum culture was positive for Mycobacterium tuberculosis complex, which was sensitive to all tuberculostatic agents. Treatment with antituberculous drugs was maintained, with good tolerance and favourable clinical course. Antiretroviral therapy was begun. The patient was discharged from the hospital 40 days after admission. The follow-up chest radiography obtained after one month of antituberculous therapy showed resolution of opacities (Figure 2).
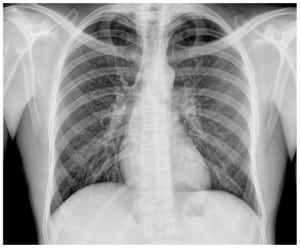
DISCUSSION
Miliary tuberculosis is an extrapulmonary form of tuberculosis due to hematogenous dissemination of Mycobacterium tuberculosis, and occurs more frequently in infants, young children, elderly subjects and immunocompromised patients. Miliary shadowing was present in 29% of patients with disseminated tuberculosis.3 Extrapulmonary tuberculosis has become more common since the advent of HIV infection. HIV is a recognised risk factor for extrapulmonary tuberculosis.4 In adults, miliary tuberculosis may be secondary to a recent infection or due to reactivation of an old focus. Miliary dissemination occurs in 10% of HIV-infected patients and up to 38% in patients with HIV and extrapulmonary tuberculosis.5 Clinical manifestations are non-specific and varied in relation to the predominant localization, including severe presenting forms with septic shock and respiratory insufficiency. Symptoms include fever, nocturnal sweating, anorexia and weight loss.6 In patients with respiratory involvement, cough and other respiratory symptoms are present. In about 85% of patients, the chest radiograph shows a reticulonodular pattern with nodules of 2–3 mm in diameter.5,6 The diagnosis may require sputum smear studies, fiberoptic bronchoscopy with bronchoalveolar lavage, blood cultures for Mycobacterium spp. or culture and histological detection of granulomas (caseous necrosis) in liver, lung or bone marrow biopsies.5,6 Moreover, in case of HIV-infected patients with advanced immunosuppression, a differential diagnosis with other opportunistic infections, like Pneumocystis jiroveci pneumonia, should be established. In these patients, the diagnosis of miliary tuberculosis when the acid-fast stain is negative requires a high index of clinical suspicion.
In the case here reported, two aspects should be highlighted: firstly, the fact that positive bacilloscopy is exceptionally recorded in a patients with a miliary radiological pattern without pulmonary infiltrates, and secondly, the severity of the tuberculosis infectious process in a patient with HIV coinfection. Septic shock associated with miliary tuberculosis in HIV infection has a high mortality, but in this case, treatment was successful and allowed complete resolution of radiological findings.
In the last decades, a better clinical and immunological control of HIV-infected patients following the use of highly-active antiretroviral treatment has been associated with a decrease in the incidence of opportunistic infections, including infections caused by Mycobacterium spp.7,8 However, in recent years, resurgence of these infections in the Western world (United States9, United Kingdom, Sweden10, Spain,2,11) have occurred probably in relation to the high immigration fluxes of people from countries with a high prevalence of tuberculosis. It is reported that many immigrants may be carriers of latent tuberculosis infection; a positive tuberculin test is found in 34.3–48.2% of the immigrant population, which is higher than rates found in the Spanish population.12,13 In addition, the prevalence of latent tuberculosis in immigrants is related to the country of origin and its socioeconomic level, with higher rates among people from Asia, Central Africa, South Africa and Eastern Europe than in those from Latin America, North African countries and Commonwealth countries.12 Extrapulmonary tuberculosis is more frequent in immigrant populations in some Western countries. Therefore, the relative increase in extrapulmonary forms of tuberculosis in these countries may be related to high rates of tuberculosis in immigrants.12,13 Pleura and lymph nodes are the most frequent extrapulmonary sites in immunocompetent subjects, whereas miliary and lymph node involvement are most frequent in immunocompromised patients.15
In summary, extrapulmonary and disseminated forms of tuberculosis infection may occur in our environment in relation to an increase in the immigrant population, occasionally associated with low socioeconomic status. These forms may have atypical presenting manifestations that together with cultural and idiomatic barriers may be difficult to diagnose and delay a correct diagnosis of tuberculosis.
Footnotes
We would like to thank M. Pulido for editorial assistance.
Supervising Section Editor: Tareg Bey, MD
Submission history: Submitted November 12, 2009; Revision Received: March 18, 2010; Accepted May 6, 2010
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Address for Correspondence: August Supervía, MD, PhD, Department of Emergency Medicine. Hospital del Mar, Ps Marítim 25-29, 08003, Barcelona, Spain
Email: Asupervia@imas.imim.es
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Kindelán JM, Natera C. Tuberculosis en grupos de riesgo. Inf Ter Sist Nac Salud. 2006;30:3–10.
2. Grupo de Trabajo de los Talleres de 2001 y 2002 de la Unidad de Investigación en Tuberculosis de Barcelona. Prevention and control of imported tuberculosis. Med Clin (Barc) 2003;121:549–62.[PubMed]
3. Crump JA, Reller LB. Two decades of disseminated tuberculosis at a University Medical Center: the expanding role of Mycobacterial blood culture. Clin Infect Dis. 2003;37:1037–43. [PubMed]
4. Yang Z, Kong Y, Wilson F, et al. Identification of risk factors for extrapulmonary tuberculosis. Clin Infect Dis. 2004;38:199–205. [PubMed]
5. Golden MP, Vikram HR. Extrapulmonary tuberculosis: and overview. Am Fam Physician.2005;72:1761–8. [PubMed]
6. Ruíz-Manzano J, Blanquer R, Calpe JL, et al. Recomendations of the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR). Diagnosis and treatment of tuberculosis. Arch Bronconeumol. 2008;44:551–66. [PubMed]
7. Detels R, Tarwater P, Phair JP, et al. Multicenter AIDS Cohort Study. Effectiveness of potent antiretroviral therapies on the incidence of opportunistic infections before and after AIDS diagnosis.AIDS. 2001;15:347–55. [PubMed]
8. Kirk O, Gatell JM, Mocroft A, et al. Infections with mycobacterium tuberculosis and mycobacterium avium among HIV-infected patients after the introduction of highly active antiretroviral therapy. EuroSIDA Study Group JD. Am J Respir Crit Care Med. 2000;162:865–72.[PubMed]
9. American thoracic society documents. America thoracic society/centres for disease control and prevention/infectious diseases society of America: controlling tuberculosis in the United States. Am J Respir Crit Care Med. 2005;172:1169–227. [PubMed]
10. Resport in tuberculosis cases notified in 2005. Institut de veille sanitaire; Saint-Maurice. France: Mar, 2007. Euro TB and the national coordinators for tuberculosis surveillance in the WHO European Region. Surveillance of tuberculosis in Europe.
11. Supervía A, Del Baño F, López Casanova MJ, et al. Characteristics of patients with tuberculosis admitted from emergency department in a University Hospital: comparative study 1996–2006. Med Clin (Barc) 2008;131:237. [PubMed]
12. Alcaide Megías J, Altet Gómez MN, Souza Galvao ML, et al. Active tuberculosis case finding among immigrants in Barcelona. Arch Bronconeumol. 2004;40:453–8. [PubMed]
13. Bran C, Gómez i Prat J, et al. Factors associated with latent tuberculous infection in immigrants less than 35 years old. Enferm Infecc Microbiol Clin. 2006;24:322–5. [PubMed]
14. García JF. Extrapulmonary tuberculosis: situation in a new century. Enferm Infecc Microbiol Clin. 2008;26:537–9. [PubMed]
15. Kipp AM, Stout JE, Hamilton CD, et al. Extrapulmonary tuberculosis, human immunodeficiency virus, and foreign birth in North Carolina, 1993–2006. BMC Public Health. 2008;8:107.[PMC free article] [PubMed]


