| Author | Affiliation |
|---|---|
| Filiberto Zadini, MD | Northridge Hospital, Northridge California |
| Edward Newton, MD | University of Southern California – Keck School of Medicine, Department of Emergency Medicine |
| Amin A. Abdi, BS | University of Southern California – Keck School of Medicine, Department of Emergency Medicine |
| Jay Lenker, PhD | MicroVention, Inc, Aliso Viejo, CA |
| Giorgio Zadini, MD | California Hospital Medical Center, Los Angeles, CA |
| Sean O. Henderson, MD | University of Southern California – Keck School of Medicine, Department of Emergency Medicine |
ABSTRACT
Introduction:
Cardiopulmonary resuscitation (CPR) is now widely used as a treatment for ventricular fibrillation, though numerous studies have shown the outcome of standard CPR to be dismal. Alternative methods of CPR, including interposed abdominal compression, constant aortic occlusion, and the use of intrathoracic pressure regulator, have been shown to increase cardiac output and affect the mortality rate of CPR. Here we suggest the Trendelenburg position as yet another method of increasing cardiac output and therefore improving the effectiveness of chest compressions. We hypothesized that the use of the Trendelenburg position during CPR would increase cardiac output as measured by carotid blood flow.
Methods:
We anaesthetized six pigs and measured their pre-arrest carotid flow rate for two minutes. We then induced ventricular fibrillation in those pigs and performed open-chest CPR on them. Post-arrest carotid blood flow was measured for two minutes each at 0 (supine position), 10, 20, and 30 degrees of head-down tilt in each pig. The mean carotid flow for each degree of tilt was compared to mean carotid flow at 0 degrees of tilt using a paired student t-test.
Results:
We found an increase of up to 1.4-fold in carotid blood flow during CPR in the Trendelenburg position, though only 20 and 30 degrees of Trendelenburg showed a statistically significant increase from the 0 degrees of tilt in pigs.
Conclusion:
The Trendelenburg position can lead to increased blood flow through the carotid arteries during CPR in this pig model. Future studies should investigate whether this increased blood flow through the carotid arteries leads to improved brain perfusion and better neurologic outcomes.
INTRODUCTION
Approximately 225,000 people in the United States and 375,000 people in Europe experience cardiac arrest every year, the majority of which occur in an out-of-hospital setting.1,2 Cardiac arrest occurs in the form of ventricular fibrillation (VF), which is treatable by shock administration, or as pulseless electrical activity (PEA) or asystole, which are not treatable through shock administration. The degree of cardiac and neurological damage following VF is correlated with the amount of blood flow through coronary and carotid arteries respectively. In situations when immediate defibrillation is not an option, cardiopulmonary resuscitation (CPR) should be initiated in order to temporarily maintain enough flow to the heart and the brain.3–8
There are many factors that can contribute to a depressed cardiac output during CPR. For example, it has been suggested that the average rescuer performing CPR can achieve adequate depth of compression in majority of cases on women but only in small percentage of men who comprise the majority of cardiac arrest victims.9 The inadequate compression depth would decrease the CPR-generated cardiac output. In addition, incomplete chest decompression during CPR performed by a fatigued rescuer can increase the intrathoracic pressure, impair venous return to the heart, and decrease cardiac output as well as coronary and cerebral perfusion pressures.10 Therefore, there is a need to further improve CPR-generated cardiac output and cerebral perfusion in order to compensate for such lack of compression depth and incomplete decompression. Compared to standard CPR there is an increase in cardiac output and blood flow to the heart and brain with methods such as the use of an intrathoracic pressure regulator,11,12 interposed abdominal compression,13–15 and constant aortic occlusion.16,17
We would like to propose the Trendelenburg position as yet another adjunct to CPR. The Trendelenburg position was once advocated to increase cardiac filling in low-flow states such as hypotension and shock. However, multiple studies were unable to demonstrate this hypothesized benefit.18–23 This lack of clinical benefit may be due to the vasoconstriction and increased arteriolar vascular tone seen during the compensation stage of hypovolemic shock, which would counteract the effects of placing the patient in the Trendelenburg position. However, during cardiac arrest, the victim has no tone and one would expect the model to change. In this study we used a porcine model to measure carotid blood flow as an indirect measure of cardiac output during experimental CPR and compared this flow for varying degrees of Trendelenburg positioning.
METHODS
This study was conducted at Biosurg, Inc. in Winters, California with adherence to existing protocols. All work was performed in accordance with the “Guide for the Care and Use of Laboratory Animals” prepared by the National Research Council of the National Institutes of Health.
Animal Preparation
Six adult pigs weighing 80 to 100 kilograms were studied. All the animals received halothane and isofluorane anesthesia. They were intubated with ventilation established before being placed supine on the operating table. We obtained right carotid access using a cutdown procedure. A 4 mm non-cannulating flow probe was attached to the right common carotid artery. To allow placement of other monitoring devices we performed a median sternotomy to provide complete access to the heart, while keeping the pericardium intact over the ventricles. ECG leads were attached for continuous monitoring. A 5 French pressure measuring catheter was advanced into the femoral artery via cutdown and positioned in the aortic arch. A capnograph was set to operate from a transducer in the endotracheal tube. Flow and pressure and capnograph devices were zeroed and calibrated. The ventilation depth and rate were adjusted to achieve between 30 and 35 mmHg end-tidal carbon dioxide measurement (pETCO2). The carotid flow rate, the ECG, and the pETCO2 were obtained continuously from this point until the end of the study.
Experimental Protocol
Before inducing VF, the pre-arrest carotid flow rate for each animal was measured at 0 degrees of tilt (supine position) in order to compare with the post-arrest carotid flow rates. Each pig was then placed into VF by direct application of current from a 9-volt battery. A minimally invasive device was advanced against the pericardium. To standardize the CPR performed on each animal, the minimally invasive device was pumped at 90 to 110 beats per minute at 10 to 12 pounds of force. The minimally invasive device is made up of an 8 cm diameter expandable membrane at the end of a shaft. Initially, the membrane is collapsed within a 45 French access sheath to allow easier introduction into the subject’s thorax. There is a 67 French flange in position on the sheath handle to prevent over-penetration of the device into the thorax.
We obtained baseline post-arrest carotid flow rates for two minutes with the animal at supine position. The table was then subsequently tilted to 10, 20, and 30 degrees of incline with the head placed down, and two minutes of data were collected for each degree of incline. We collected hemodynamic data at least every 30 seconds at each angle of tilt. Each animal served as its own control as it was placed in increased Trendelenburg tilts in a sequential manner, and its mean carotid flow rate was compared to both the pre-arrest and post-arrest baseline values. At the end of the experimental period, the animals were euthanized by termination of circulatory support.
Statistical Methods
We analyzed the significance of hemodynamic changes with degree of head-down tilt using a PC-based statistical analysis package. A paired student t-test was used as each animal served as its own control. Statistical significance was accepted if p < 0.05. The coefficient of determination r2 was determined using the same software.
RESULTS
The mean carotid flow in the pre-arrest phase of the study was 442 mL/min. After induction of ventricular fibrillation with CPR in progress this decreased to 149 mL/min (difference of 293 mL/min (95% CI: 188, 398)).
For 10 degrees of incline, only three complete sets of data were available due to technical issues with the flow meters of the other animals. These three animals had a post-arrest mean carotid flow of 165 mL/min at 0 degrees of tilt that increased to 177 mL/min at 10 degrees of tilt (an increase of 12mL/min from baseline (95% CI: −12,35)).
At 20 degrees of incline, six complete sets of data were available. The post-arrest mean carotid flow for all six pigs at this level of incline was 194 mL/min (an increase of 46 mL/min from baseline (95% CI of the difference: 23, 69)).
For 30 degrees of incline, four complete sets of data were available. These four animals had a post-arrest mean carotid flow of 128 mL/min at 0 degrees of tilt and 178 mL/min at 30 degrees of tilt (an increase of 50 mL/min from baseline (95% CI: 9, 91)) (Table 1) (Figure 1a).
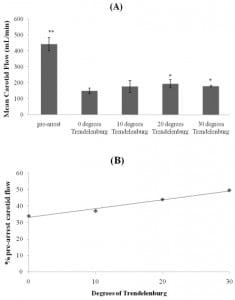
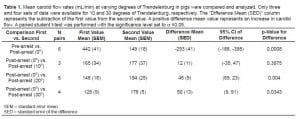
Compared to baseline CPR at supine position, compressions at head-down angles of 10, 20 and 30 degrees resulted in 1.1, 1.3, and 1.4 times greater carotid flow, respectively. An alternate method of viewing the data is as a percentage of the mean carotid flow in the pre-arrest phase. The mean carotid flow at 0 degrees was 34% of pre-arrest flow. At 10 degrees of Trendelenburg the mean flow increased to 37% of the pre-arrest value, at 20 degrees thisvaluewas. The 10 degree of 44%, and at 30 degrees of tilt the mean carotid flow was 49.5% of pre-arrest value. The change in percentage of pre-arrest flow rates were tightly associated with the degrees of Trendelenburg tilt with a coefficient of determination r2= 0.98 (Figure 1b).
DISCUSSION
Initially developed as a surgical position, the Trendelenburg position was later adopted by Walter Cannon as a treatment for shock during World War I. Although it has been widely taught and used by healthcare professionals,24 its effectiveness as a treatment for shock has not yet been clinically shown despite numerous studies. The Trendelenburg position does not have any beneficial hemodynamic effects in hypotensive patients,18,19 leads to no changes in tissue oxygenation in hypovolemic postoperative patients,20 and appears to cause small and ineffective autotransfusion changes in normovolemic patients.21 Reich and colleagues22 report an increase in mean arterial pressure and cardiac output in hypotensive patients with coronary artery disease following placement in the Trendelenburg position, although this position led to impaired respiration in those patients to a greater extent. Furthermore, steep head-down positions have been reported to have potentially harmful effects in patients with cardiac, respiratory, and neural problems. Such effects include increased intracranial pressure and respiratory compromise.23 There has also been a report of brachial plexus injury in a post-operative patient who underwent a long period of head-down tilt at an angle of 30–40 degrees.25
With the use of Trendelenburg positioning during CPR we were able to see an increase in carotid blood flow. Our results show that the use of this position improved carotid blood flow during CPR in this porcine model. We found the 20 and 30 degrees of tilt to have a statistically significant higher carotid flow. The 10 degree of head-down tilt also showed an increased carotid blood flow, although this increase was not statistically significant. Chest compressions while placing the animals in the Trendelenburg position produced up to 1.4-fold increase in the carotid flow compared to the standard CPR. It is suggested that other adjuncts to conventional CPR also result in improved circulation during cardiac arrest. Gedeborg and colleagues16 reported an increase of 62% in carotid blood flow following balloon occlusion of descending aorta, and Yannopoulos and colleagues12reported an average increase of 70% in carotid artery flow with the use of an intrathoracic pressure regulator during CPR. Similarly, Babbs13 suggested that by combining interposed abdominal compression and chest compression the total cardiac output may show a 1.9-fold increase compared to conventional CPR alone. These adjuncts, however, have their own limitations; the use of constant aortic occlusion requires a period of conventional CPR while the balloon catheter is inserted,16 and interposed abdominal compression requires the availability of additional rescuer personnel as well as further training.13 Nonetheless, recent studies have suggested these adjuncts to be effective and their combination with the use of the Trendelenburg position may prove to be beneficial.
One of the major goals of cardiopulmonary resuscitation is to preserve neurological function, which in turn is dependent on the perfusion of cerebral tissue. The brain is very vulnerable to hypoxic/ischemic injury. Following cardiac arrest and CPR, approximately 80% of patients are comatose with about 40% entering persistent vegetative state.26Novel approaches, such as mild therapeutic hypothermia (lowering body temperature to 33°C for 12 to 24 hours following return of spontaneous circulation), have been suggested to help with ischemic brain injury, and recent studies have shown an increase in favorable neurological outcomes following therapeutic hypothermia after resuscitation from cardiac arrest.1,2
Many authors have also suggested adjuncts to standard CPR to improve outcomes, including the use of intraortic balloon pumps, abdominal binding systems and counterpulsation techniques, either through abdominal compression or timed respirations. All of these adjuncts attempt to improve blood flow during standard closed-chest CPR by increasing the venous return to the thorax and heart (i.e. prime the pump).13–17, 27–28 Given that cardiac output is a function of both the stroke volume and the rate, and that the slow ventricular filling during CPR limits the rate of effective compressions, the purpose of a good adjunct should be to increase the stroke volume in the face of a constant compression rate.
An increase in carotid blood flow through the use of Trendelenburg position is an important finding with clinical implications. An increase in carotid blood flow may improve cerebral oxygen delivery during CPR and, together with the use of mild therapeutic hypothermia, may further improve neurological outcomes following resuscitation. However, it must be noted that the “Guidelines for Cardiopulmonary Resuscitation” recommends placing, the post-cardiac arrest survivors in a 30 degrees reverse Trendelenburg position with head at midline.29 This position is thought to maximize cerebral arterial perfusion without compromising cerebral venous drainage after resuscitation, though its effectiveness has not yet been studied.30 Therefore, placing the patient in the Trendelenburg position may theoretically lead to an increase in the intracranial pressure during cardiopulmonary resuscitation, which can lead to a decrease in cerebral perfusion—the opposite of what is intended by this maneuver. This requires additional studying and is further discussed in the limitations section of our study.
Although numerous studies of the Trendelenburg position in healthy volunteers and hospitalized patients have suggested little or no beneficial hemodynamic effects, it may well be beneficial during CPR. The ability of an individual, healthy or hypotensive, to autoregulate peripheral and central vascular tone (specifically, the arteriolar vascular tone) minimizes the effect that a head-down position might have on cerebral blood flow.31On the other hand, a person experiencing cardiac arrest with concomitant decrease in vascular tone has lost such autoregulatory reflexes. The head-down position during cardiac arrest may lead to increased arteriolar blood flow with the aid of gravity, as the vascular tone is decreased due to the loss of autoregulatory reflexes. Our findings support this theory as we saw an increase in CPR-generated carotid blood flow with increasing Trendelenburg.
At the same time it must be noted that the venous system is not significantly affected by the autoregulatory reflexes, considering the lower vascular tone of a venule compared to an arteriole. Yet the application of Trendelenburg could simply aid the return of the pooled venous blood from the periphery to the heart and in this way serve as the means to increase the cardiac output through increasing the preload. However, one can argue that more blood flow to the arrested heart could lead to a higher right atrial pressure and a subsequent decrease in coronary perfusion pressure (defined as the difference between diastolic aortic pressure and right atrial pressure)—another unwanted effect. Future experiments should investigate these possibilities in more detail.
LIMITATIONS
It is important to note the following limitations of our study. Firstly, the sample size was small. Secondly, venous return to the heart was not measured, and although it is believed that the Trendelenburg position would lead to increased venous flow to the heart, there is no direct evidence for that. Thirdly, our study employed an open-chest CPR technique, which may compromise any benefit of the thoracic pump model of blood flow during closed chest CPR. Regardless, open-chest CPR has been repeatedly shown to be superior to closed-chest CPR based upon standard measures of arterial pressure generated, cardiac output, coronary perfusion pressure and cerebral blood flowachieved.32–33 Fourthly, the applicability of the pig as a model for CPR has been shown, but the appropriateness of the pig to simulate the relative effects of Trendelenburg in arrest with the comparatively small leg volume of the pig cannot be predicted. Fifthly, because return of spontaneous circulation and the neurologic outcome of CPR with Trendelenburg were not measured in this particular model there is no information as to the overall effect of this increased cerebral flow on survivors of cardiopulmonary arrest. Sixthly, though carotid blood flow was used as an indirect measure of cardiac output and cerebral perfusion, direct measurements of intracranial pressure, cerebral perfusion pressure and actual cerebral blood flow were not performed. Theoretically, if the head-down position augments forward flow, cerebral venous return through internal jugular veins may be proportionately diminished due to gravity. This may lead to decreased delivery of waste products from the CNS, as well as increased intracranial pressure and decreased cerebral perfusion pressure, negating the effects of the increased carotid flow. Furthermore, during placement in the Trendelenburg position the abdominal organs can be pushed into the thoracic cavity increasing the intrathoracic pressure. Recent studies suggest that an increase in the intrathoracic pressure would lead to an increase in the intracranial pressure, decreasing cerebral perfusion.10–12 However, a modified Trendelenburg position with cervical flexion and elevation of the head might minimize these potential negative effects without diminishing the forward flow through the carotids. It is also possible that the increased carotid flow simply reflects the backflow of blood from the abdominal aorta as an effect of gravitational forces, and this may lead to decreased perfusion of other organs, including the kidneys and intestines. Finally, the pigs were moved from 0 to 10 to 20 to 30 degrees in a step-wise fashion, possibly exaggerating the effects of each position.
CONCLUSION
Future studies should be undertaken to: 1) investigate the efficacy of Trendelenburg position on a closed-chest CPR technique; 2) determine the effects of both standard Trendelenburg and modified Trendelenburg with cervical flexion on venous return to the heart, intracranial pressure, coronary and cerebral perfusion pressures, as well as internal jugular vein and abdominal aorta blood flow to control for the negative effects of gravity; 3) investigate return to spontaneous circulation and neurologic outcome of concomitant CPR and Trendelenburg; 4) determine flow at an initial setting of 20 to 30 degrees in the immediate post-arrest phase.
Despite the limitations of the study, in all of the animals studied carotid flow increased as the animal was placed into the Trendelenburg position. Our findings indicate that 20 and 30 degrees of head-down tilt can improve carotid flow in pigs in a statistically significant manner. Such findings merit further investigations of the use of Trendelenburg position during CPR. Whether these findings can be extrapolated to human subjects undergoing CPR remains to be seen.
Footnotes
Supervising Section Editor: Jeffrey Sankoff, MD
Submission history: Submitted October 31, 2007; Revision Received July 15, 2008; Accepted August 4, 2008
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Address for correspondence: Sean O. Henderson, MD. Department of Emergency Medicine, LAC+USC Medical Center, Unit #1, Room 1011, 1200 N. State Street, Los Angeles, CA 90033
Email: sohender@hsc.usc.edu
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Curfman GD. Hypothermia to protect the brain. Perspective. N Engl J Med.2002;346:546. [PubMed]
2. The Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556.[PubMed]
3. Steen S, Liao Q, Pierre L, Paskevicius A, Sjöberg T. The critical importance of minimal delay between chest compressions and subsequent defibrillation: haemodynamic explanation. Resuscitation. 2003;58:249–258. [PubMed]
4. Kern KB, Ewy GA, Voorhees WD, Babbs Cf, Tacker WA. Myocardial perfusion pressure: a predictor of 24-hour survival during prolonged cardiac arrest in dogs. Resuscitation.1988;16:241–250. [PubMed]
5. Michael JR, Guerci AD, Koehler RC, Shi AY, Tsitlik J, Chandra N, Niedermeyer E, Rogers MC, Traystman RJ, Weisfeldt ML. Mechanisms by which epinephrine augments cerebral and myocardial perfusion during cardiopulmonary resuscitation in dogs.Circulation. 1984;69:822–835. [PubMed]
6. Paradis NA, Martin GB, Rivers EP, Goetting Mg, Appleton TJ, Feingold M, Nowak RM. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA. 1990;263:1106–1113. [PubMed]
7. von Plata I, Weil MH, von Plata M, Bisera J, Bruno S, Gazmuri RJ, Rackow EC. Cardiopulmonary resuscitation in the rat. J Appl Physiol. 1988;65:2641–2647.[PubMed]
8. Andreka P, Frenneaux MP. Haemodynamics of cardiac arrest and resuscitation.Current opinion in critical care. 2006;12:198–203. [PubMed]
9. Rottenberg EM. The need for a leftward shift in the flow-depth relationship during cardiopulmonary resuscitation. Resuscitation. 2007;72:350–352. [PubMed]
10. Yannopoulos D, McKnite S, Aufderheide TP, Sigurdsson G, Pirrallo RG, Benditt D, Lurie KG. Effects of incomplete chest wall decompression during cardiopulmonary resuscitation on coronary and cerebral perfusion pressure in a porcine model of cardiac arrest.Resuscitation. 2005;64:363–372. [PubMed]
11. Yannopoulos D, Nadkarni VM, McKnite S, Rao A, Kruger K, Metzger A, Benditt D, Lurie KG. Intrathoracic pressure regulator during continuous-chest-compression advanced cardiac resuscitation improves vital organ perfusion pressures in a porcine model of cardiac arrest. Circulation. 2005;112:803–811. [PubMed]
12. Yannopoulos D, Aufderheide TP, McKnite S, Kotsifas K, Charris R, Nadkarni V, Lurie KG. Hemodynamic and respiratory effect of negative tracheal pressure during CPR in pigs. Resuscitation. 2006;69:487–494. [PubMed]
13. Babbs CF. Efficacy of interposed abdominal compression-cardiopulmonary resuscitation (CPR), active compression and decompression-CPR, and Lifestick CPR: Basic physiology in a spreadsheet model. Crit Care Med. 2000;28:N199–N202. [PubMed]
14. Babbs CF. Interposed abdominal compression CPR: a comprehensive evidence based review. Resuscitation. 2003;59:71–82. [PubMed]
15. Xavier L, Kern KB, Berg RA, Hilwig RW, Ewy GA. Comparison of standard CPR versus diffuse and stacked hand position interposed abdominal compression-CPR in a swine model. Resuscitation. 2003;59:337–344. [PubMed]
16. Gedeborg R, Rubertsson S, Wiklund L. Improved haemodynamics and restoration of spontaneous circulation with constant aortic occlusion during experimental cardiopulmonary resuscitation. Resuscitation. 1999;40:171–180. [PubMed]
17. Gedeborg R, Silander HC, Rubertsson S, Wiklund L. Cerebral ischaemia in experimental cardiopulmonary resuscitation—comparison of epinephrine and aortic occlusion. Resuscitation. 2001;50:319–329. [PubMed]
18. Taylor J, Weil MH. Failure of the Trendelenburg position to improve circulation during clinical shock. Surg Gynecol Obstet. 1967;124:1005–1010. [PubMed]
19. Sibbald WJ, Paterson NA, Holliday RL, Baskerville J. The Trendelenburg position: hemodynamic effects in hypotensive and normotensive patients. Crit Care Med.1979;7:218–224. [PubMed]
20. Sing RF, O’Hara D, Sawyer MA, Marino PL. Trendelenburg position and oxygen transport in hypovolemic adults. Ann Emerg Med. 1994;23:564–567. [PubMed]
21. Bivins HG, Knopp R, dos Santos PA. Blood volume distribution in the Trendelenburg position. Ann Emerg Med. 1985;14:641–643. [PubMed]
22. Reich DL, Konstadt SN, Hubbard M, Thys DM. Do Trendelenburg and passive leg raising improve cardiac performance? . Anesth Analg. 1988;67:S184.
23. Johnson S, Henderson SO. Myth: the Trendelenburg position improves circulation in cases of shock. Canadian Journal of Emergency Medicine. 2004;6:48–49. [PubMed]
24. Ostrow CL. Use of the Trendelenburg position by critical care nurses: Trendelenburg survey. Am J Crit Care. 1997;6:172–176. [PubMed]
25. Craig J. Shoulder supports, brachial plexus injury and head-down tilt. Anaesthesia.2004;59:196. [PubMed]
26. Madl C, Holzer M. Brain function after resuscitation from cardiac arrest. Current Opinion in Critical Care. 2004;10:213–217. [PubMed]
27. Koehler RC, Chandra N, Guerci AD, Tsitlik J, Traystman RJ, Rogers MC, Weisfeldt ML. Augmentation of cerebral perfusion by simultaneous chest compression and lung inflation with abdominal binding after cardiac arrest in dogs. Circulation. 1983;67:266–275. [PubMed]
28. Wenzel V, Lindner KH, Prengel AW, Strohmenger HU. Effect of phased chest and abdominal compression-decompression cardiopulmonary resuscitation on myocardial and cerebral blood flow in pigs. Crit Care Med. 2000;28:1107–1112. [PubMed]
29. Advances in cardiovascular life support—section 8: post-resuscitation care.Circulation. 2000;102(suppl I):I166–I177. [PubMed]
30. Wright WL, Geocadin RG. Postresuscitative intensive care: neuroprotective strategies after cardiac arrest. Seminars in neurology. 2006;26:396–402. [PubMed]
31. Hu Z, Zhao G, Xiao Z, Chen X, Zhong C, Yang J. Different responses of cerebral vessels to -30 degrees head-down tilt in humans. Aviat Space Environ Med. 1999;70:674–680.[PubMed]
32. Alzaga-Fernandez AG, Varon J. Open-chest cardiopulmonary resuscitation: past, present and future. Resuscitation. 2005;64:149–156. [PubMed]
33. Benson DM, O’Neil B, Kadish E, Erpelding J, Alousi S, Mason R, Piper D, Rafols J. Open-chest CPR improves survival and neurologic outcome following cardiac arrest.Resuscitation. 2005;64:209–217. [PubMed]


