| Author | Affiliation |
|---|---|
| Robin A. Marshall, MD | Naval Medical Center Portsmouth, Department of Emergency Medicine, Portsmouth, VA |
| Chris Hejamanowski, MD | Naval Medical Center Portsmouth, Department of Emergency Medicine, Portsmouth, VA |
ABSTRACT
Introduction:
Determining the presence or absence of red blood cells (RBC) or their breakdown products in cerebrospinal fluid (CSF) is essential for the evaluation of subarachnoid hemorrhage (SAH) in headache patients. Current methodology for finding blood in the CSF is either spectrophotometric detection of pigment, which is time consuming and labor intensive, or visual assesment of samples for color change (xanthochromia), which is inaccurate. Bayer Multistix® urine test strips are designed to test urine for RBC by detecting the presence of hemoglobin. The aim of this pilot study was to evaluate the perfomance of urine reagent test strips for ruling out the presence of RBC in CSF.
Methods:
We compared color changes on Multistix® urine test strips to the standard of spectrophotometric absorbtion at 415nm and initial RBC counts in 138 visually clear CSF samples.
Results:
We performed Pearson Chi-Square and likelihood ratios on the results and found a correlation between a negative result on the urine test strip and less than 5 RBC per high power field and a spectrophotometric absorbance of less than 0.02% at 415nm in a CSF sample.
Conclusion:
These results warrant further investigation in the form of a prospective clinical validation as it may alter the emergency department evaluation for SAH.
INTRODUCTION
Patients presenting to the emergency department complaining of rapid onset or “thunderclap” headache require an urgent clinical evaluation to rule out a subarachnoid hemorrhage (SAH). This process typically involves a non-contrast head computed tomography and, if negative, a lumbar puncture to determine the presence of blood or blood products in the cerebrospinal fluid (CSF). The characteristic, faint yellow staining of CSF by RBC breakdown products is known as xanthochromia. When red blood cells (RBC) lyse in CSF they release hemoglobin that is later enzymatically converted to methemoglobin and bilirubin. The number of intact blood cells is typically determined by either an automated cell counter or by counting cells per high power field in manual microscopy. Most United States laboratories currently determine xanthochromia by using a visual testing method in which a sample of CSF is compared to a white background and any staining present is noted. Unfortunately results can be highly inaccurate if the visual testing method is employed.1 The more accurate but less common method for determining xanthochromia is to use a spectrophotometer and record a sample absorbance at wavelengths known to correspond with breakdown pigments, such as hemoglobin, methemoglobin and bilirubin. Laboratory evaluation of CSF can require one to two hours or more to complete if spectrophotometric methods are used. Quickly and reliably confirming the lack of clinically significant levels of RBC in CSF might significantly expedite the evaluation of these headache patients. When evaluating a patient for the possibility of SAH a negative reagent test for blood might obviate further lab investigation or imaging. A straightforward, rapid reagent test has the potential to save clinical time and diagnostic costs.
Other medical settings in which reliable, expeditious CSF results would be valuable are those with less access to technology and resources. Across Third World nations and in military Combat Support Hospitals, patients requiring evaluations for headaches may not have access to radiology, photo spectrometry, microscopy or automated cell counters. In these austere settings, visually ruling out xanthrochromia becomes paramount. A reagent test that capitalizes on simplicity and economy would better serve these resource-constrained facilities.
METHODS
Research data were derived as an approved study under the supervision of the local Institutional Review Board/Institutional Animal Care and Use Committee protocol (CIP# 2006.0027). We conducted a laboratory evaluation from July to October 2006, using CSF samples submitted to the hospital laboratory. In the vast majority of cases the volume of CSF submitted to a lab is more than is needed for the requested diagnostic evaluation. This study intercepted this surplus CSF prior to disposal. The CSF was stored for up to one week at 4°C until analyzed, then allowed to warm to room temperature prior to testing. We inspected all samples to eliminate those with any visually discernible xanthochromia. This visual sorting was undertaken to narrow our test pool only to samples that would have been classified as negative using the common practice methods in place in most labs. A visually pigmented sample needs no further evaluation as it is already positive. The investigators found 138 samples to be visually clear.
We placed each clear sample placed in a Bausch & Lomb™ Spectronic 601 spectrophotometer and recorded the percent light absorption at 415 nm. Xanthochromia is considered present in CSF when spectrophotometric absorbance ≥0.024% at 415nm. Graves et al2 has recently suggested a more conservative value of ≥0.023%. We used a cutoff of ≥0.02% at 415nm to provide a safety margin and to reflect the trend toward a more stringent threshold for xanthochromia.
A different investigator used a Bayer Multistix® urine test strip to test the same sample for the presence of blood. Following the manufacturer’s procedure for urine testing, we placed a drop of sample CSF on the blood square on the urine test strip. At 60 seconds, we noted and compared the color change against the key found on the side of the container. Since the CSF used in this study were samples that had already been analyzed by the hospital lab, at the end of each testing session we looked up and recorded each sample’s cell counts after visual sorting, spectrophotometry and urine test strip analysis was completed. We then compared the urine dipstick testing results to the CSF blood cell counts and spectrophotometric results for statistical analysis.
We maintained a pooled sample of CSF known to be free of red cells and tested it biweekly with urine strips to ensure that the test strips would not yield false positive results due to aging CSF or other unanticipated factors. This true negative standard was made from CSF samples that had shown no absorbance on spectrophotometry, had tested negative on the test strips, and did not contain greater than five red cells per high power field. We used this standard as a measure to ensure that true negative results on the test strips were reliable, reproducible, and durable.
RESULTS
Since the goal of this project was to use urine test strips to rule out the presence of red cells in CSF, we distributed the results into two groups. Group one consisted of samples with negative urine dip results. Group two was comprised of samples showing any positive dip result. We considered a sample positive if there was any color change on the test strip, regardless of the magnitude or pattern of the color change. Sixty-three of 138 samples tested negative for blood using the urine dipstick method.
We assigned spectrophotometer results above the cutoff to one group, below to a second. Using spectrophotometric methods, we found that 103 of 138 samples tested negative for xanthochromia.
The microscopic analysis results for RBCs per high power field were assigned to one of three groups: 0–5, 6–10, and >10. We chose two cutoffs for red blood cells (5/hpf and 10/hpf) to represent common lab thresholds for “normal” red cells in CSF. Ninety-nine samples had ≤5 rbc/hpf, 12 samples had 5–10 rbc/hpf, and 27 samples were >10 rbc/hpf. The Multistix® proved to be very sensitive. Of samples with zero to five cells per high power field, 37 showed some positive results. When a threshold of zero cells per high power field was used, 15 out of 138 samples were false positive.
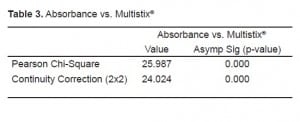
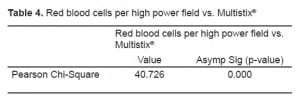
We conducted two Pearson Chi-Square tests. The first compared samples above and below the spectrophotometric cutoff to samples with negative and non-negative results on the urine test strips (Table 1). The second compared the three sets of red cell results to negative and non-negative results on the urine test strips (Table 2). We found significant correlation (p<0.0001) between a negative result on the urine test strip and the negative cutoff of 0.020% spectrophotometric absorption at 415nm. We found this same correlation (P<0.0001) between a negative test strip result and a CSF microscopic reading <5 RBC/HPF. Tables 3 and and44 depict the statistical results.
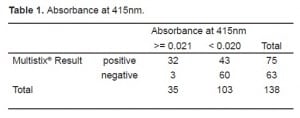
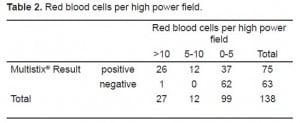
When compared to spectrophotometry, the Multistix® had a sensitivity of 0.91 and a negative predictive value of 0.95, with a specificity of only 0.58. The negative likelihood ratio was 0.16. When compared to the RBC/HPF (cutoff of ≤5), the Multistix® had a sensitivity of 0.97, a negative predictive value of 0.98, with a specificity of 0.62. The negative likelihood ratio was 0.04. We used the highly conservative spectrophotometric cutoff value of 0.020 for this analysis; only further work will determine if this cutoff was too conservative.
The true negative pooled CSF yielded no false positive reaction on the Multistix® during the four-month period of this study.
DISCUSSION
A SAH occurs with the release of blood into the CSF. It subsequently breaks down over time into the characteristic pigments of hemoglobin, bilirubin and methhemoglobin. Hemoglobin from red cell lysis is the first significant compound, presenting as early as two hours, peaking at around 24 hours, and persisting for up to a week. Bilirubin is only created in vivo and much later than hemoglobin, as its creation requires the action of an arachnoid oxygenase not present in the test tube once a sample is collected, making it much more difficult to study. Methemoglobin represents another RBC breakdown product that, like bilirubin, is only encountered after time. Typically patients with severe headaches present acutely, often within a few hours of onset of their symptoms. This makes hemoglobin the best target pigment for this pilot work in test strip diagnosis of blood in the CSF. As Multistix® include a Bilirubin test block, subsequent clinical exploration should include this pigment.
We were concerned about the number of samples that were visually pigment free, per the inclusion criteria for this study, but which subsequent microscopic or spectrophotometric analysis indicated were positive for blood. These findings support other authors’1, 3, 4 misgivings about using visually determined xanthochromic absence to rule out intrathecal blood. Unfortunately, testing for xanthochromia using visual methodology is still employed by many hospitals, particularly in the United States. Should urine test strip results correlate well with spectrophotometric results, they would provide for a quick, easy alternative to visual xanthochromia determination in centers without access to spectrophotometery.
An examination of the data reveals almost two thirds of the visually clear samples tested negative for blood via reagent strips. Hence the statistical reassignment was undertaken. Our goal was to evaluate the performance of the Multistix® in a Boolean, yes/no paradigm. In other words, we wanted to know how good a negative result on the Multistix® was. When compared to the gold standard of spectrophotometery for xanthochromia, the Multistix® showed promise as a simple test to rule out red cells in the CSF. SAH is a life-threatening condition. High test sensitivity is essential in a screening test for detecting of intrathecal blood. With a high sensitivity a resultant increase in false positive results is to be expected. The false positive rate would need to be closely monitored if this diagnostic method were to be evaluated in a clinical setting. It may subsequently be shown that CSF testing with urine test strips is only useful when the result is negative.
As the focus of this study was a bench-top evaluation of the performance of urine test strips in analyzing CSF, the circumstances and settings under which we obtained these samples were of secondary importance. Often novel diagnostic strategies are evaluated directly in a clinical setting without first determining whether they perform in a controlled laboratory setting.
Our use of an all comers convenience sample implies that some of the CSF we analyzed was obtained from patients in whom other diagnoses, such as infection rather than subarachnoid hemorrhage, were being entertained. Might there be some protein or entity present during an infection and not present during simple hemorrhage that would yield a false positive result? Bayer assured the investigators that the “heme” portion of the Multistix® was specific for blood. Unfortunately the exact mechanism of the colorimetric reaction is understandably proprietary. Multistix® detect hemoglobin in the cell; therefore, it can be inferred that the process of spotting samples onto urine reagent strips lyses red cells to release their contents for subsequent detection by colorimetric chemical reaction. This lysis effectively removes the potential confound of early tap analyzed prior to cell lysis and release of pigment.
LIMITATIONS
This is a relatively small, bench-top pilot study; no clinical implications are suggested or warranted without proper prospective validation.
Traumatic tap rates are reported as high as 10%.3 As urine test strips most likely lyse cells in their detection of pigment, they would be unlikely to assist with detecting a traumatic tap caused by the presence of hemoglobin. Bilirubin theoretically shows more promise for differentiating traumatic lumbar puncture from subarachnoid hemorrhage, with the important caveat that it would only be expected to be present at 12 hours after the onset of bleeding.4 As this study used lab surplus CSF samples of varying ages, we did not assess the performance of the Bilirubin panel on the urine test strips.
The use of surplus samples made it impossible to control for the clinical setting of the lumbar puncture. These samples left us unable to ascertain the indications for the procedure or the technique employed to obtain the samples. Some authors have suggested that using Betadine® as a skin prep can introduce spurious coloration to a sample.5 While it is unlikely that storage of CSF affected the results, this study’s use of lab samples represents a potential confound that can only be addressed by a clinical trail.
CONCLUSION
Given a correlation between a negative result on the urine test strip and less than five RBC per high power field and a spectrophotometric absorbance of less than 0.02% at 415nm in a CSF sample, determining the absence of CSF RBCs via benchtop CSF evaluation of urine test strips shows enough promise to warrant further study in a prospective clinical setting. Ultimately this may prove to be an expeditious and cost-effective adjunct to the evaluation of headaches when ruling out subarachnoid hemorrhage.
Footnotes
Supervising Section Editor: Teresita M Hogan, MD
Submission history: Submitted: October 6, 2009; Revision received April 4, 2010; Accepted April 19, 2010.
Reprints available through open access at http://escholarship.org/uc/uciem_westjem
Address for Correspondence: Robin A Marshall, MD, FAAEM, Department of Emergency Medicine, Naval Medical Center Portsmouth, Portsmouth, VA 23708
Email: robin.marshall@med.navy.mil
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
Disclosures: The authors have no financial or proprietary interests to disclose. I am an employee of the U.S. government. This work was prepared as part of my official duties. Title 17 U.S.C. 105 provides that ‘Copyright protection under this title is not available for any work of the United States Government.’ Title 17 U.S.C. 101 defines a United States Government work as a work prepared by a military service member or employee of the United States Government as part of that person’s official duties. Research data derived from Ruling out CS blood using urine reagent testing strips, an approved Naval Medical Center, Portsmouth, VA IRB/IACUC protocol (CIP#2006.0027).
REFERENCES
1. Petzold A, Keir G, Sharpe TL. Why human color vision cannot reliably detect cerebrospinal fluid xanthochromia. Stroke. 2005;36:1295–97. [PubMed]
2. Graves P, Sidman R. Xanthochromia is not pathognomonic for subarachnoid hemorrhage. Acad Emerg Med. 2004;11:131–5. [PubMed]
3. Mark DG, Pines JM. The detection of nontraumatic subarachnoid hemorrhage: still a diagnostic challenge. Am J Emerg Med. 2006;24:859–63. [PubMed]
4. van Gijn J. Slip-ups in diagnosis of subarachnoid haemorrhage. Lancet. 1997 May 24;349:1492.[PubMed]
5. Iversen SA. CSF spectrophotometry in the diagnosis of subarachnoid haemorrhage. J Clin Pathol.2002;55:640. [PMC free article] [PubMed]


