| Author | Affiliation |
|---|---|
| Joel M. Schofer, MD | Naval Medical Center, Portsmouth, VA |
| Jason T. Nomura, MD | Christiana Care Health System, Department of Emergency Medicine, Newark, DE |
| Michael J. Bauman, MD | Christiana Care Health System, Department of Emergency Medicine, Newark, DE |
| Paul R. Sierzenski, MD | Christiana Care Health System, Department of Emergency Medicine, Newark, DE |
ABSTRACT
Introduction:
We assessed the acoustic transmission, image quality, and vessel integrity of the Blue Phantom™ 2 Vessel Original Ultrasound Training Model with repeated use.
Methods:
The study consisted of two phases. During the first phase, a portion of the Blue Phantom™ rubber matrix (without a simulated vessel) was placed over a two-tiered echogenic structure and was repeatedly punctured with a hollow bore 18-gauge needle in a 1 cm2 area. During the second phase, a portion of the matrix with a simulated vessel was repeatedly punctured with another hollow bore 18-gauge needle. During both phases we obtained an ultrasound image using a high-frequency linear probe after every 100 needle punctures to assess the effect of repeated needle punctures on image quality, acoustic transmission, and simulated vessel integrity.
Results:
Testing on the rubber matrix alone (first phase) without a vessel demonstrated a gradual decrease in image quality and visualization of the proximal and distal portions of the target structure, but they remained visible after 1,000 needle punctures. The second phase demonstrated excellent acoustic transmission and image quality on both transverse and longitudinal images of the rubber matrix and simulated vessel after 1,000 needle punctures. The anterior and posterior vessel walls and needle tip were well visualized without any signs of vessel leakage on still images or with compression and power Doppler.
Conclusion:
The Blue Phantom™ 2 Vessel Original Ultrasound Training Model demonstrated excellent durability after 1,000 needle punctures in a 1- cm2 area. Based on the length of simulated vessel in each model, it should support over 25,000 simulated attempts at vascular access.
INTRODUCTION
A large body of medical research advocates the use of ultrasound guidance when obtaining central and peripheral vascular access.1–18 In addition, major governmental organizations have recommended using ultrasound guidance when obtaining central venous access.19,20
The increasing use of ultrasound guidance for vascular access has created an educational need. Vascular access phantoms that mimic human soft tissue and vascular structures allow for ultrasound-guided vascular access training without exposing patients to painful, risky procedures. While private corporations have begun producing these vascular access phantoms, they are often expensive and have not been subject to independent testing to ensure durability with repeated use.
The Blue Phantom™ (Kirkland, WA) 2 Vessel Original Ultrasound Training Model is commonly used to teach ultrasound-guided peripheral vascular access and therefore was selected for durability testing. It consists of a rubber matrix and fluid-filled tubes simulating human soft tissue and peripheral vascular structures, respectively. We conducted independent durability testing to assess its acoustic transmission, image quality, and vessel integrity with repeated use.
METHODS
The study, approved by the local institutional review committee consisted of two phases. During the first phase a portion of the Blue Phantom™ rubber matrix (without a simulated vessel) was placed over an echogenic structure, in a water bath, and repeatedly punctured with a hollow bore 18-gauge needle in a 1 cm2 area. We did this to test the acoustic transmission of the rubber matrix and visibility of the echogenic structure with repeated punctures. During the second phase we repeatedly punctured a portion of the matrix with a simulated vessel with another hollow bore 18-gauge needle in a 1 cm2 area at a 45° angle. The simulated vessel was assessed for fluid leakage with active compression and power Doppler.
During both phases we obtained an ultrasound image using a Sonosite™ (Bothell, WA) M-Turbo ultrasound machine with a 25mm, 6–13 MHz linear probe after every 100 needle punctures to assess the effect of repeated needle punctures on image quality, acoustic transmission, and simulated vessel integrity. All settings (depth, gain, frequency, etc.) were unchanged during the acquisition of images. All images were obtained and later assessed qualitatively in digital format in an unblinded fashion by four board certified/eligible emergency physicians who were in an emergency ultrasound fellowship or had completed a fellowship. Both phases of the study were concluded after a total of 1,000 needle punctures.
RESULTS
The first phase of testing on the rubber matrix alone without a vessel demonstrated a gradual decrease in image quality and visualization of the proximal and distal portions of the target structure, but they remained visible after 1,000 needle punctures. (Figures 1–2)
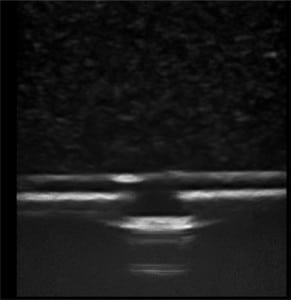
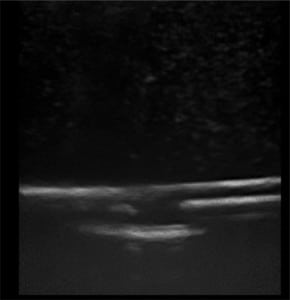
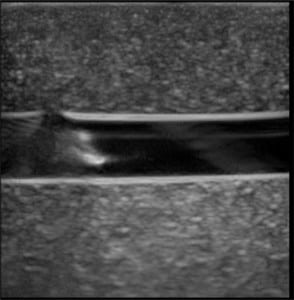
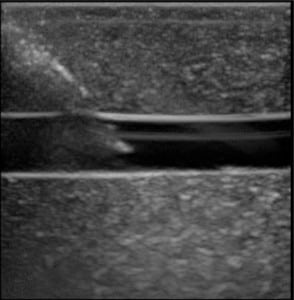
The second phase of the study demonstrated excellent acoustic transmission and image quality on both short- and long-axis images of the rubber matrix and simulated vessel after 1,000 needle punctures. The anterior and posterior vessel walls and needle tip were well visualized without any signs of vessel leakage on still images or with compression and power Doppler. (Figures 3–6)
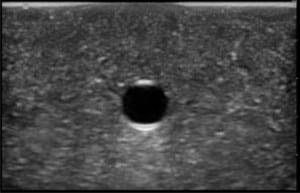
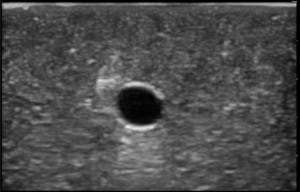
DISCUSSION
The Blue Phantom™ 2 Vessel Original Ultrasound Training Model demonstrated excellent durability after 1,000 needle punctures in a 1 cm2 area. The rubber matrix demonstrated a gradual decrease in acoustic transmission, but this did not affect the ability to visualize the anterior and posterior walls of the simulated vessel or the needle tip. The integrity of the simulated vessel was well preserved without any signs of vessel leakage.
LIMITATIONS
This study tested only one of the many different commercially available vascular access phantoms; therefore, the results may not be applicable to other products on the market. In addition, because we only punctured the simulated vessel and rubber matrix with an 18-gauge needle, these results may not be reproduced if a different needle size or a catheter/needle combination is used. Despite the manufacturer’s recommendation against this, some users who access the simulated vessel aspirate and then re-inject the fluid into the vessel, which often leads to air deposits in the phantom material. This practice and the air deposited can lead to more rapid image degradation than was seen in our study. Further research could address these variables.
CONCLUSION
If the full length of simulated vessel contained in this vascular access phantom is used (excluding the vessel on the ends of the phantom), each model should support over 25,000 simulated attempts at vascular access without significant degradation in the integrity of the simulated vessel or the ultrasound image produced.
Footnotes
The Blue Phantom™ 2 Vessel Original Ultrasound Training Model was provided by the manufacturer for testing. They had no input into the study design or analysis of the results.
I am a military service member. This work was prepared as part of my official duties. Title 17, USC, 105 provides that ‘Copyright protection under this title is not available for any work of the United States Government.’ Title 17, USC, 101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, or the United States government.
Supervising Section Editor: J Christian Fox, MD
Submission history: Submitted July 26, 2009; Revision Received January 24, 2010; Accepted February 22, 2010
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Address for Correspondence: Joel M. Schofer, MD, 3713 Farnsworth Dr., Chesapeake, VA 23321
Email: jschofer@gmail.com
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Arun Prasad G, Niazi A, Chan V. Ultrasound averts inadvertent injury during internal jugular vein cannulation. Can J Anaesth. 2009;56:85–6. [PubMed]
2. Bair AE, Rose JS, Vance CW, et al. Ultrasound-assisted peripheral venous access in young children: A randomized controlled trial and pilot feasibility study. WestJEM. 2008;9:219–24.[PMC free article] [PubMed]
3. Brannam L, Blaivas M, Lyon M, et al. Emergency nurses’ utilization of ultrasound guidance for placement of peripheral intravenous lines in difficult-access patients. Acad Emerg Med.2004;11:1361–3. [PubMed]
4. Caridi JG, Hawkins IF, Jr, Wiechmann BN, et al. Sonographic guidance when using the right internal jugular vein for central vein access. AJR Am J Roentgenol. 1998;171:1259–63. [PubMed]
5. Conz PA, Dissegna D, Rodighiero MP, et al. Cannulation of the internal jugular vein: Comparison of the classic seldinger technique and an ultrasound guided method. J Nephrol. 1997;10:311–3.[PubMed]
6. Costantino TG, Parikh AK, Satz WA, et al. Ultrasonography-guided peripheral intravenous access versus traditional approaches in patients with difficult intravenous access. Ann Emerg Med.2005;46:456–61. [PubMed]
7. Gallieni M, Cozzolino M. Uncomplicated central vein catheterization of high risk patients with real time ultrasound guidance. Int J Artif Organs. 1995;18:117–21. [PubMed]
8. Hilty WM, Hudson PA, Levitt MA, et al. Real-time ultrasound-guided femoral vein catheterization during cardiopulmonary resuscitation. Ann Emerg Med. 1997;29:331–6. discussion 337. [PubMed]
9. Hudson PA, Rose JS. Real-time ultrasound guided internal jugular vein catheterization in the emergency department. Am J Emerg Med. 1997;15:79–82. [PubMed]
10. Keyes LE, Frazee BW, Snoey ER, et al. Ultrasound-guided brachial and basilic vein cannulation in emergency department patients with difficult intravenous access. Ann Emerg Med. 1999;34:711–4.[PubMed]
11. Lamperti M, Caldiroli D, Cortellazzi P, et al. Safety and efficacy of ultrasound assistance during internal jugular vein cannulation in neurosurgical infants. Intensive Care Med. 2008;34:2100–5.[PubMed]
12. Lee W, Leduc L, Cotton DB. Ultrasonographic guidance for central venous access during pregnancy. Am J Obstet Gynecol. 1989;161:1012–3. [PubMed]
13. Leung J, Duffy M, Finckh A. Real-time ultrasonographically-guided internal jugular vein catheterization in the emergency department increases success rates and reduces complications: A randomized, prospective study. Ann Emerg Med. 2006;48:540–7. [PubMed]
14. Miller AH, Roth BA, Mills TJ, et al. Ultrasound guidance versus the landmark technique for the placement of central venous catheters in the emergency department. Acad Emerg Med.2002;9:800–5. [PubMed]
15. Milling TJ, Jr, Rose J, Briggs WM, et al. Randomized, controlled clinical trial of point-of-care limited ultrasonography assistance of central venous cannulation: The third sonography outcomes assessment program (SOAP-3) trial. Crit Care Med. 2005;33:1764–9. [PubMed]
16. Pirotte T. Ultrasound-guided vascular access in adults and children: Beyond the internal jugular vein puncture. Acta Anaesth Belg. 2008;59:157–66. [PubMed]
17. Shiver S, Blaivas M, Lyon M. A prospective comparison of ultrasound-guided and blindly placed radial arterial catheters. Acad Emerg Med. 2006;13:1275–9. [PubMed]
18. Slama M, Novara A, Safavian A, et al. Improvement of internal jugular vein cannulation using an ultrasound-guided technique. Intensive Care Med. 1997;23:916–9. [PubMed]
19. Technology appraisal guidance no. 49 – guidance of the use of ultrasound locating devices for placing central venous catheters. 2002. pp. 1–21.
20. Shojania KG, Duncan BW, McDonald KM, et al. Making health care safer: A critical analysis of patient safety practices. Evidence Report/Technology Assessment No 43 (Prepared by the University of California at San Francisco–Stanford Evidence-based PracticeCenter under Contract No 290-97-0013), AHRQ Publication No 01-E058. 2001. pp. 245–54.


