| Author | Affiliation |
|---|---|
| David R. Vinson, MD | The Permanente Medical Group, Oakland, California; Kaiser Permanente Roseville Medical Center, Roseville, California |
| Dustin W. Ballard, MD, MBE | The Permanente Medical Group, Oakland, California; Kaiser Permanente San Rafael Medical Center, San Rafael, California |
| Matthew D. Stevenson, BS | Loma Linda University School of Medicine, Loma Linda, California |
| Dustin G. Mark, MD | The Permanente Medical Group, Oakland, California; Kaiser Permanente Oakland Medical Center, Oakland, California |
| Mary E. Reed, DrPH | Kaiser Permanente Division of Research, Oakland, California |
| Adina S. Rauchwerger, MPH | Kaiser Permanente Division of Research, Oakland, California |
| Uli K. Chettipally, MD, MPH | The Permanente Medical Group, Oakland, California; Kaiser Permanente South San Francisco Medical Center, South San Francisco, California |
| Steven R. Offerman, MD | The Permanente Medical Group, Oakland, California; Kaiser Permanente South Sacramento Medical Center, Sacramento, California |
| For the Kaiser Permanente CREST Network Investigators |
Introduction
Methods
Results
Discussion
Limitations
Conclusion
ABSTRACT
Introduction
Central venous catheterization (CVC) can be an important component of the management of patients with severe sepsis and septic shock. CVC, however, is a time- and resource-intensive procedure associated with serious complications. The effects of the absence of shock or the presence of relative contraindications on undertaking central line placement in septic emergency department (ED) patients eligible for early goal-directed therapy (EGDT) have not been well described. We sought to determine the association of relative normotension (sustained systolic blood pressure >90 mmHg independent of or in response to an initial crystalloid resuscitation of 20 mL/kg), obesity (body mass index [BMI] ≥30), moderate thrombocytopenia (platelet count <50,000 per μL), and coagulopathy (international normalized ratio ≥2.0) with unattempted CVC in EGDT-eligible patients.
Methods
This was a retrospective cohort study of 421 adults who met EGDT criteria in 5 community EDs over a period of 13 months. We compared patients with attempted thoracic (internal jugular or subclavian) CVC with those who did not undergo an attempted thoracic line. We also compared patients with any attempted CVC (either thoracic or femoral) with those who did not undergo any attempted central line. We used multivariate logistic regression analysis to calculate adjusted odd ratios (AORs).
Results
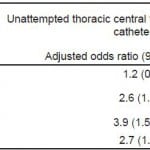
In our study, 364 (86.5%) patients underwent attempted thoracic CVC and 57 (13.5%) did not. Relative normotension was significantly associated with unattempted thoracic CVC (AOR 2.6 95% confidence interval [CI], 1.6–4.3), as were moderate thrombocytopenia (AOR 3.9; 95% CI, 1.5–10.1) and coagulopathy (AOR 2.7; 95% CI, 1.3–5.6). When assessing for attempted catheterization of any central venous site (thoracic or femoral), 382 (90.7%) patients underwent attempted catheterization and 39 (9.3%) patients did not. Relative normotension (AOR 2.3; 95% CI, 1.2–4.5) and moderate thrombocytopenia (AOR 3.9; 95% CI, 1.5–10.3) were significantly associated with unattempted CVC, whereas coagulopathy was not (AOR 0.6; 95% CI, 0.2–1.8). Obesity was not significantly associated with unattempted CVC, either thoracic in location or at any site.
Conclusion
Septic patients eligible for EGDT with relative normotension and those with moderate thrombocytopenia were less likely to undergo attempted CVC at any site. Those with coagulopathy were also less likely to undergo attempted thoracic central line placement. Knowledge of the decision-making calculus at play for physicians considering central venous catheterization in this population can help inform physician education and performance improvement programs.
INTRODUCTION
Central venous catheterization can play a critical role in the management of patients with severe sepsis and septic shock.1–3 Central venous access is necessary for the administration of vasopressors, which can be damaging to smaller peripheral veins and result in extensive tissue necrosis in the event of extravasation. Catheterization of a thoracic central vein, either the internal jugular or subclavian, also allows the direct measurement of central venous pressure and central venous oxygen saturation. Abnormalities of these measures can be used to grade the severity of sepsis and their normalization can serve as a goal of resuscitation.4
Thoracic central venous catheterization, however, is a time- and resource-intensive procedure associated with serious mechanical complications, including pneumothorax and hemorrhage. Thoracic central venous catheterization has been identified by both physicians and nurses in busy urban emergency departments (EDs) as one of several barriers to the implementation of national sepsis treatment guidelines.5,6
The decision to pursue central venous catheterization for the administration of vasopressors may seem more compelling than when the line’s only purpose is directing protocolized management. In the latter case, especially, weighing indications and relative contraindications can be a difficult calculus. This is due to the fact that the precise incremental benefit of a thoracic central line in early goal-directed therapy (EGDT) among various subpopulations of septic patients has yet to be quantified. It is unclear how much weight should be given to various relative contraindications to central line placement. For example, the risk of complications with thoracic central venous catheterization in septic patients with abnormal hemostasis in an age of ultrasound guidance is not well characterized. Absent evidence of this kind, physicians are guided by clinical judgment informed by training, experience, and local practice patterns.7 How this works out in clinical practice in terms of procedures attempted and procedures averted has not been described. We undertook this cohort study of septic ED patients eligible for EGDT to determine to what extent, if at all, relative normotension, obesity, and abnormal hemostasis were associated with failure to attempt central venous catheterization.
METHODS
Study Setting and Design
We analyzed a cohort of adult septic patients who met criteria for EGDT between August 1, 2009, and August 31, 2010, in 5 community EDs within Kaiser Permanente Northern California (KPNC). KPNC is a large integrated healthcare delivery system that provides comprehensive care for more than 3.4 million members and receives over 900,000 annual ED visits. KPNC health plan members represent approximately 25–30% of the population in areas served and are similar to the general population with respect to race/ethnicity, socioeconomic status, and education, with the exception of a slight underrepresentation of the extremes of income.8,9 The study was reviewed and granted formal exemption by the KPNC Health Services Institutional Review Board.
The study EDs are staffed by emergency medicine residency-trained and board-certified (or board-eligible) physicians. The departments vary in volume. During the study period, 3 EDs each had an approximate annual census of 75,000. The other 2 had an annual census of 35,000 and 25,000, respectively. Two of the 5 EDs are affiliated with a university emergency medicine residency training program. One ED is a Level II trauma center. All medical centers have adult intensive care units with bed capability ranging from 12 to 32.
The study period followed the implementation of a standardized version of EGDT as part of a region-wide quality improvement initiative that included a training program at each facility on sepsis diagnoses, management, and ultrasound-guided thoracic central venous catheterization. The other components of our medical group’s performance improvement program have been described elsewhere.10 Sepsis management during the study period followed a modified Rivers protocol that did not require arterial catheterization.4 The modified protocol also allowed ScvO2 monitoring to occur continuously through a specialized ScvO2 catheter or intermittently through centrally drawn venous blood gases.
We explore unattempted thoracic central venous catheterization, because EGDT calls for thoracic line placement. But we know from experience that physicians who avoid placing a thoracic central line for whatever reason may nonetheless attempt femoral venous catheterization. We chose therefore to study patient variables associated with both unattempted thoracic central vein catheterization as well as unattempted placement at any site, thoracic or femoral.
We hypothesized that 3 patient variables might prove a significant deterrent to thoracic central venous catheterization even when otherwise clinically indicated and encouraged by an active quality improvement program: (1) relative normotension, which might imply that thoracic central venous access was not really necessary despite a serum lactate level ≥4 mmol/L; (2) obesity, which might dissuade a physician from attempting such a procedure because of its perceived technical difficulty; and (3) abnormal hemostasis (either moderate thrombocytopenia or coagulopathy), which might suggest that the risk of bleeding from a venous (or accidental arterial) puncture is greater than the benefit gained from thoracic central venous access.
We assumed that in higher risk situations the clinical decision making would tilt more favorably toward femoral venous access than thoracic venous access because femoral lines might be perceived to be less technically difficult to accomplish and easier to directly compress in the case of excessive post-procedural bleeding. We hypothesized then that obesity and abnormal hemostasis would not be associated with unattempted central venous access when attempted femoral vein catheterization was included in the analysis.
Relative normotension in this study is defined as a sustained systolic blood pressure (SBP) >90 mmHg, either independent of or in response to initial fluid resuscitation of 20 mL/kg of intravenous crystalloids over one hour. Obesity is defined as a body mass index (BMI) ≥30. Moderate thrombocytopenia is defined as an ED platelet count <50,000/μl. An ED international normalized ratio (INR) ≥2.0 constitutes coagulopathy. The latter 2 are referred to as disorders of hemostasis.
Selection of Participants
We identified the cohort from a larger KPNC sepsis database (the Quality database) created retrospectively and managed by the data consulting team of the Quality and Accreditation, Regulation and Licensing Division of Kaiser Foundation Hospital, Inc. We included adult non-gravid patients (≥18 years of age) from KPNC’s 21 EDs in the Quality database if they had an inpatient diagnosis of severe sepsis or septic shock (ICD-9 codes: 003.1, 036.2, 038.0–038.9, 785.52, 995.91, 995.92), major infection in the ED (known or suspected), and either 2 or more systemic inflammatory response syndrome (SIRS) criteria or an altered level of consciousness.10 Excluded were ED patients with comfort care status, those admitted directly to the operating suite, and patients with hypotension or lactate elevation that the treating emergency physician (EP) ascribed to a non-infectious etiology, e.g., a patient with a massive lower gastrointestinal bleed without evidence of infection.
Our study cohort was a subpopulation of the Quality database, limited to patients with severe sepsis or septic shock treated at 1 of the 5 study EDs during the study period. We excluded from the cohort patients who had declined central venous catheterization (either directly or through their caregiver or family), as well as those with a pre-existing thoracic central venous catheter or port.
Patients who met eligibility criteria were then categorized for study purposes as having severe sepsis or septic shock as follows: patients in the severe sepsis category had metabolic evidence of tissue hypoperfusion (an elevated ED serum lactate level ≥4 mmol/L) combined with relative normotension (defined above). Patients in the septic shock category had refractory hypotension (a SBP ≤90 mmHg that failed with initial volume resuscitation to stay above the 90 mmHg threshold), regardless of the ED serum lactate value.
Methods and Measurements
Two investigators (MDS, DRV) used a computerized data abstraction tool to abstract demographic, clinical, and management variables from the electronic health records. After confirming the patient’s study eligibility, we collected the following variables related to the index ED visit: patient age, sex, weight, date and site of ED visit, and SBP, both initially and after initial fluid resuscitation. Patient weight was taken in nearly all cases from measurements obtained either at the time of the ED admission (often for stable ambulatory patients) or during the inpatient intake assessment (particularly for unstable and non-ambulatory patients). In only a few cases, when a measured weight was not identified, it was taken at face value from the patient or family report.11,12 Values obtained through electronic databases included patient height, initial ED serum lactate level, initial ED platelet count, and initial ED International Normalized Ratio (INR) (when performed). Missing values are reported as such.
The primary outcome of interest was attempted central venous catheterization (either thoracic or any site) during the ED stay. We reviewed EP and nursing notes for documentation of attempted central venous access. We also reviewed radiology reports of ED chest radiographs for evidence of films ordered to assess for post-procedural complications, as patients with an attempt (successful or not) at thoracic central line placement routinely undergo chest radiographs to detect iatrogenic pneumothorax. To reduce abstraction bias, both abstractors confirmed eligibility on all cases. Both abstractors also reviewed and confirmed all cases that failed to receive an attempted central line. A third investigator arbitrated any ambiguities encountered during electronic chart review (e.g., in eligibility, sepsis classification, or central line attempts).
Statistical Analysis
Continuous variables are presented as means with standard deviation and categorical data are presented as the percentage of frequency of occurrence (p-values are shown for t-test or chi-squared test). A p-value of less than 0.05 was considered to indicate statistical significance. We performed bivariate analysis to compare patients with attempted central venous catheterization with patients without attempted central venous catheterization. Adjusted odds ratios were calculated using multivariate logistic regression to determine independent predictors of unattempted thoracic and any-site central line placement. The 4 variables that drove our hypotheses (BMI ≥30, SBP >90 mmHg, platelet count <50,000 per μL, and INR ≥2.0) were included in the model. Standard errors in the model were adjusted for clustering by attending physician. We conducted sensitivity analysis among patients without repeated ED visits during the study period and found comparable results. We also conducted sensitivity analysis by including age and gender in the regression models, as well as by changing the BMI cut-off to ≥40 or excluding BMI altogether. With all these analyses we found the results to be comparable, i.e., these changes did not affect the direction or statistical significance of the findings. We included missing responses as a separate category for each variable. Analyses were performed using Stata statistical software, version 10 (StataCorp LP, College Station, TX).
RESULTS
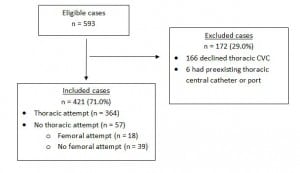
During the 13-month study period, 593 septic ED patients were recognized by their EPs in the study EDs as having a known or suspected major infection and met eligibility criteria for EGDT. Of these, 166 (28.0%) declined central venous catheterization and 6 (1.0%) patients had a pre-existing central vein access port, leaving 421 patients in the cohort.
One hundred fifty-one (35.9%) had severe sepsis and 270 (64.1%) had septic shock as previously defined. (See Figure for the flow of patients). Overall, 226 (53.7%) were men; mean age was 66 ± 16.1 years (range 18–96). The sources of sepsis were as follows: pulmonary 192 (45.6%); urinary 91 (21.6%); intra-abdominal 43 (10.2%); skin/soft tissue 24 (5.7%); other 71 (16.8%). Of the total cohort, 364 (86.5%) patients underwent attempted thoracic central venous catheterization and 57 (13.5%) patients did not. Of these 57 patients, 18 (31.6%) underwent attempted femoral venous catheterization, leaving 39 patients who did not undergo an attempt at either thoracic or femoral central venous catheterization.
Figure. Flow of study patients. CVC, central venous catheterization
Demographic and clinical characteristics of the patients are shown in Table 1. The groups were comparable in bivariate analysis in age, sex, mean BMI, mean serum lactate level, and mean platelet count. The only variables with missing values were BMI (11 [2.6%] patients had no height recorded in the medical record) and INR (155 [36.8%] patients did not have INR measured in the ED). Missing values for these two variables were equally distributed between the groups.
Table 1. Characteristics of septic emergency department patients eligible for early goal-directed therapy (n=421).
*Thoracic central venous catheterization includes access via the internal jugular or subclavian veins.
†Systolic blood pressure >90 mmHg either independent of or in response to initial crystalloid bolus of 20 mL/kg.
We found that relative normotension, moderate thrombocytopenia, and INR≥2 were significantly associated with unattempted thoracic central venous catheterization (see Table 2). With regard to any-site access, relative normotension and moderate thrombocytopenia were associated with unattempted catheterization, but an elevated INR was not (Table 2).
Table 2. Adjusted associations between patient characteristics and unattempted thoracic central venous catheterization in septic emergency department patients eligible for early goal-directed therapy (n=421).
*We included in the analysis an indicator for missing values.
†Either independent of or in response to initial crystalloid bolus of 20 ml/kg
‡p<0.001 §p<0.05 ||p<0.01
Fourteen patients of the cohort met eligibility criteria on 2 different dates throughout the study period and were included in the analysis. Since each visit represented a different medical decision-making process about risks and benefits of central line placement, they were retained in the study. We adjusted for clustering of patients using sensitivity analysis and found comparable results. Likewise, the results were comparable when these 14 second visits were dropped entirely from analysis.
Seventeen patients met diagnostic criteria for septic shock in the ED but failed to receive attempted ventral venous catheterization during their ED stay. The probable causes were as follows: immediate transfer to the intensive care unit where a central line would be placed in a timely fashion (n=2), awaiting response to ED blood transfusion (n=2), disorders of hemostasis (n=6), transient SBP response to volume resuscitation (n=4), and continued fluid administration despite failure of SBP response (n=3).
DISCUSSION
This multi-center cohort study found that septic ED patients eligible for EGDT are less likely to undergo attempted thoracic central venous catheterization when relatively normotensive or when presenting with moderate thrombocytopenia (platelet count <50,000/mL) or coagulopathy (INR≥2.0). Also, septic patients are less likely to undergo central venous catheterization at any site, thoracic or femoral, when relatively normotensive or when presenting with moderate thrombocytopenia. Identifying which patient variables are associated with procedural avoidance helps demonstrate how physicians calculate the risk/benefit ratio when weighing explicit indications against relative contraindications for internal jugular, subclavian, and femoral venous catheterization.
We found that EGDT-eligible patients with sustained relative normotension (following volume resuscitation if indicated) were less likely to receive attempted central venous access. This result is consistent with Mikkelson et al13 who found in multivariable analysis that normal blood pressure was independently associated with a failure to initiate EGDT. Similarly, Kakebeeke et al14 reported that septic ED patients with only biochemical signs of organ failure, i.e., hyperlactatemia, were less likely to receive the full recommended resuscitation bundle compared with those who had overt, clinically recognizable signs of organ failure, i.e., hypotension. The disinclination to attempt an invasive procedure in normotensive patients with severe sepsis who are not in overt shock could be attributable to the generally less ill appearance of this population. It could be that the clinical gestalt of the physicians tells them the central venous catheter may be unnecessary to the resuscitation since vasopressors are unlikely to be indicated.15 Anecdotal reports suggest this is true. Further stratification of the ED sepsis population may well demonstrate that a one-sized approach does not fit all comers.16
Irrespective of the need for vasopressors, the EGDT protocol for sepsis management calls for thoracic central venous catheterization in order to measure and monitor central venous oxygen saturation and central venous pressure. But recent research in noninvasive approaches to resuscitation monitoring suggests that central venous catheterization may have fewer indications in sepsis management than proposed by the original Rivers protocol.4 Central venous pressure, as either a static or dynamic measure of intravascular volume status, has repeatedly been shown to demonstrate poor correlation with fluid responsiveness (as determined by a predetermined increase in cardiac output immediately following fluid administration).17 Lactate clearance is being explored as an alternative to central venous oxygen saturation monitoring as a marker of adequate tissue perfusion.18–22 Likewise, noninvasive assessments of intravascular volume status are being studied as alternatives to traditional invasive monitoring devices.20,23–26 Among the more promising means of detecting preload responsiveness are dynamic echocardiographic measures of cardiac output and changes in ultrasonographic venocaval dimensions in response to respirophasic physiology and passive leg raising.27
Several large, multicenter trials are currently underway that seek to clarify the role of central venous catheterization (and other components of the EGDT bundle) in the management of ED patients with severe sepsis and septic shock.28 These include the Australasian Resuscitation in Sepsis Evaluation (ARISE) trial,29 the Protocolized Care for Early Septic Shock (ProCESS) trial centered in Pittsburgh, and the Protocolised Management in Sepsis (ProMISe) trial in the United Kingdom. Perhaps select patients with relative normotension can be successfully managed without thoracic central line placement. There may be a noninvasive protocol for patients with severe sepsis soon to emerge in which EPs’ central venous catheterization hesitancy in the subpopulation with relative normotension finds justification.
This study also demonstrated that obesity, contrary to our expectation, was not significantly associated with unattempted thoracic central venous catheterization. An enlarged body habitus has historically been thought to make thoracic central venous access more difficult and dangerous, which is why obesity is often listed as a relative contraindication for this procedure. Our results, however, support a shift in perceptions and evidence. For example, prospective studies of thoracic central venous catheterization have yielded mixed results regarding BMI effects, even in those using an anatomic landmark technique. Earlier anatomic landmark studies reported that BMI extremes (either too high or too low) were associated with increased central venous catheterization complications.30,31 More recent anatomic landmark studies, however, have found that BMI had no bearing on complication rates.32,33 Several studies of the complications attending thoracic central venous catheterization have not even reported or controlled for BMI.34,35 Emergency medicine studies using real-time ultrasound guidance further support the contemporary irrelevancy of patient weight.36 Even if extremes of BMI are perceived by physicians to be associated with an increased risk of thoracic central venous catheterization failure or complications, obesity (and even morbid obesity) did not prove in our study to deter physicians from attempting thoracic catheterization when indicated. We did find suggestion of an association with obesity and unattempted central line placement at any site, although this association did not reach statistical significance.
We also found that physicians were more likely to forego attempted thoracic and femoral central line placement in septic ED patients with disorders of hemostasis, even among patients with septic shock. Moderate thrombocytopenia predicted both unattempted thoracic and any-site central venous catheterization. Coagulopathy INR (≥2.0) independently predicted unattempted thoracic venous catheterization but not any-site central venous catheterization.
It appears that EPs are prone to avoid any-site central venous catheterization in patients with moderate thrombocytopenia. Yet in patients with INR levels of 2 or greater physicians are not averse to placing a central line in general, just one located in the thoracic region. This femoral vein preference in coagulopathic patients could well be explained by the site’s easier compressibility in case of iatrogenic hemorrhage. Femoral vein access, however, is not altogether free of significant hemorrhagic complications.37–40 Why a femoral vein preference was not also observed for patients with moderate thrombocytopenia is not clear.
It seems reasonable to think that placement of a large-bore catheter into a potentially difficult-to-compress thoracic vein in patients with abnormal hemostasis would increase the risk of major hemorrhage, including intrathoracic and mediastinal bleeding. But the consensus of observational data on this topic suggests that that may not actually be the case.41–54
Though the bleeding risk increases as the platelet count drops and as the INR and partial thromboplastin time (PTT) rise, the risk remains relatively low and the bleeding complications are minor in nearly all cases. Platelet counts below 50,000/mL and an INR above the 3.0–5.0 range have been shown to confer a small risk (generally less than 5%) of minor bleeding at the catheter’s percutaneous insertion site. These local minor bleeds are most often controllable with direct pressure or a surgical stitch in the skin.43–51,53 This small risk for minor bleeds is insufficient to warrant a denial or delay in the placement of a thoracic central venous catheter when clinically indicated.
Major bleeding in these circumstances is remarkably rare. Aggregating data from 13 diverse studies over the past 30 years—some retrospective and others prospective, some using the anatomic landmark technique and other ultrasound guidance, some with residents-in-training and others with experienced clinicians—found major hemorrhage to be a rare occurence among more than 4,000 thoracic central venous catheterizations in patients with varying degrees of altered hemostasis.41–45,47–54 Nearly all of these thoracic central lines were performed without pre-procedural correction of the thrombocytopenia or coagulopathy. In fact, attempted correction of hemostatic abnormalities in patients without active bleeding may incur greater risks than benefits.42,44,55 The diverse clinical conditions represented in these studies do not directly mirror our clinical situation, however, as few patients in these case series were septic and few proceduralists were EPs. Our Kaiser Permanente CREST Network (http://www.kpcrest.net) recently completed a large retrospective cohort study of septic patients with thrombocytopenia (platelet count <100,000/mL) or coagulopathy (INR ≥1.3 or aPTT ≥35 seconds) who received central venous catheterization in the ED. Analysis of the first 700 patients, nearly all of whom received thoracic lines, suggests that major hemorrhagic events are rare; we found only one case (95% upper confidence limit: 0.8%) of major bleeding: a hemothorax from a misplaced subclavian line in a patient with an INR of 1.4.56
In light of this large body of research, moderate thrombocytopenia and coagulopathy may be less important relative contraindications for central line placement than assumed. It would follow then that the level of procedural risk aversion we demonstrated in the face of abnormal hemostasis may be overly cautious. The mortality benefit from thoracic central venous catheterization in some patients with septic shock and concomitant abnormal hemostasis is likely to outweigh the associated small risk of minor and treatable puncture-site bleeding and the very low risk of major bleeding. It has been shown that physicians are prone to overestimate risk, especially hemorrhagic risk.57 Physician education is needed to lower misinformed risk estimates of major bleeding to more accurately match evidence from the literature. Education could also address our innate omission bias, in which we are prone to more strongly avoid complications we actively cause (e.g., iatrogenic procedural bleeding) than complications we might passively allow (e.g., the increased morbidity associated with withholding central venous catheterization).57–59 The results of this study could help physicians recalculate the risk/benefit ratio of central venous catheterization in septic patients and thus recalibrate their management decisions in ways that improve their practice patterns.
LIMITATIONS
The major limitation in using health records as primary data sources for a retrospective study is missing, inconsistent, or erroneous documentation. We think the risk is negligible in regards to our dichotomous outcome measure—attempted or non-attempted central venous catheterization—since this documentation is both explicit and redundant. In addition to searching for documentation of attempted central venous access in the physicians’ notes, we also searched the nursing notes. As a third source, we reviewed the radiography reports, since post-procedural chest radiographs are ordered commonly as a matter of course to assess for iatrogenic pneumothorax in patients who undergo successful or failed thoracic central venous catheterization. Although we believe the study’s data are fairly complete and accurate, we cannot ensure the absence of error or systematic bias to which observational studies are prone.
A second limitation of this retrospective design is that other patient variables not studied herein may also predict unattempted central venous catheterization or may have confounded our associations. Also, we restricted our predictors to patient-related variables. Physician variables, such as comfort and experience with thoracic central venous access, likely influence the risk/benefit decision to attempt thoracic central venous catheterization, but are not reported in this study.
Thirdly, though we had over 400 patients in our cohort, our analysis yielded imprecise estimates, as noted by the broad confidence intervals around our adjusted odds ratios. Fourth, we do not report rates of successful line placement, use of adjunct ultrasonography, or complications of placement. Such information is interesting but beyond the scope of this study. Lastly, this study was conducted in 5 community EDs in Northern California and may not be generalized to other practices and locations. Nevertheless, we included a diversity of EDs with varying patient volumes in different cities throughout the state. Included are small and large community EDs, adjunct training centers for emergency medicine residents, and one Level II trauma center. These variations help to enhance the study’s external validity.
CONCLUSION
This multi-center cohort study found that most ED patients eligible for EGDT underwent attempted thoracic central venous catheterization. Patients with relative normotension, as well as those with abnormal hemostasis, were less likely to receive attempted central line placement, both thoracic and femoral. Knowledge of the variables associated with central venous catheterization avoidance can inform physician education and performance improvement programs on the emergency management of patients with severe sepsis and septic shock.
Footnotes
Address for Correspondence: David R. Vinson, MD. Department of Emergency Medicine, Kaiser Permanente Roseville Medical Center, Roseville, CA. Email: drvinson@ucdavis.edu. 2 / 2014; 15:67 – 75
Submission history: Revision received January 4, 2013; Submitted July 8, 2013; Accepted August 13, 2013
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. [PubMed]
2. Rivers EP, Katranji M, Jaehne KA, et al. Early interventions in severe sepsis and septic shock: a review of the evidence one decade later. Minerva Anestesiol. 2012;78:712–724. [PubMed]
3. Levinson AT, Casserly BP, Levy MM. Reducing mortality in severe sepsis and septic shock. Semin Respir Crit Care Med. 2011;32:195–205. [PubMed]
4. Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. [PubMed]
5. Burney M, Underwood J, McEvoy S, et al. Early detection and treatment of severe sepsis in the Emergency Department: Identifying barriers to implementation of a protocol-based approach. J Emerg Nurs. 2012;38:512–517. [PubMed]
6. Carlbom DJ, Rubenfeld GD. Barriers to implementing protocol-based sepsis resuscitation in the emergency department-results of a national survey. Crit Care Med. 2007;35:2525–2532. [PubMed]
7. Schwartz A, Bergus G. Medical decision making: a physician’s guide. Cambridge, UK; New York: Cambridge University Press; 2008.
8. Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82:703–710. [PMC free article][PubMed]
9. Gordon NP. Similarity of the adult Kaiser Permanente membership in Northern California to the insured and general population in Northern California: Statistics from the 2009 California Health Interview Survey. [Accessed July 3, 2013]. www.dor.kaiser.org/external/chis_non_kp_2009/
10. Whippy A, Skeath M, Crawford B, et al. Kaiser Permanente’s performance improvement system, part 3: multisite improvements in care for patients with sepsis. Jt Comm J Qual Patient Saf. 2011;37:483–493. [PubMed]
11. Corbo J, Canter M, Grinberg D, et al. Who should be estimating a patient’s weight in the emergency department? Acad Emerg Med. 2005;12:262–266. [PubMed]
12. Hall WL, 2nd, Larkin GL, Trujillo MJ, et al. Errors in weight estimation in the emergency department: comparing performance by providers and patients. J Emerg Med. 2004;27:219–224.[PubMed]
13. Mikkelsen ME, Gaieski DF, Goyal M, et al. Factors associated with nonadherence to early goal-directed therapy in the ED. Chest. 2010;138:551–558. [PMC free article] [PubMed]
14. Kakebeeke D, Vis A, de Deckere ER, et al. Lack of clinically evident signs of organ failure affects ED treatment of patients with severe sepsis. Int J Emerg Med. 2013;6:4. [PMC free article] [PubMed]
15. Schmidt GA. Counterpoint: adherence to early goal-directed therapy: does it really matter? No. Both risks and benefits require further study. Chest. 2010;138:480–3. discussion 3–4. [PubMed]
16. Perel A. Bench-to-bedside review: the initial hemodynamic resuscitation of the septic patient according to Surviving Sepsis Campaign guidelines–does one size fit all? Crit Care. 2008;12:223.[PMC free article] [PubMed]
17. Marik PE, Baram M, Vahid B. Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest. 2008;134:172–178. [PubMed]
18. Puskarich MA, Trzeciak S, Shapiro NI, et al. Prognostic value and agreement of achieving lactate clearance or central venous oxygen saturation goals during early sepsis resuscitation. Acad Emerg Med. 2012;19:252–258. [PMC free article] [PubMed]
19. Nguyen HB, Kuan WS, Batech M, et al. Outcome effectiveness of the severe sepsis resuscitation bundle with addition of lactate clearance as a bundle item: a multi-national evaluation. Crit Care. 2011;15:R229. [PMC free article] [PubMed]
20. Coen D, Vaccaro A, Cazzaniga M, et al. Toward a noninvasive approach to early goal-directed therapy. Chest. 2011;139:726–727. [PubMed]
21. Jones AE, Shapiro NI, Trzeciak S, et al. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010;303:739–746. [PMC free article][PubMed]
22. Jansen TC, van Bommel J, Schoonderbeek FJ, et al. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med. 2010;182:752–761. [PubMed]
23. Haydar SA, Moore ET, Higgins GL, 3rd, et al. Effect of bedside ultrasonography on the certainty of physician clinical decision making for septic patients in the emergency department. Ann Emerg Med. 2012;60:346–358.e4. [PubMed]
24. Wiwatworapan W, Ratanajaratroj N, Sookananchai B. Correlation between inferior vena cava diameter and central venous pressure in critically ill patients. J Med Assoc Thai. 2012;95:320–324.[PubMed]
25. Griffee MJ, Merkel MJ, Wei KS. The role of echocardiography in hemodynamic assessment of septic shock. Crit Care Clin. 2010;26:365–382. [PubMed]
26. Stawicki SP, Braslow BM, Panebianco NL, et al. Intensivist use of hand-carried ultrasonography to measure IVC collapsibility in estimating intravascular volume status: correlations with CVP. J Am Coll Surg. 2009;209:55–61. [PubMed]
27. Levitov A, Marik PE. Echocardiographic assessment of preload responsiveness in critically ill patients. Cardiol Res Pract. 2012;2012:819696. [PMC free article] [PubMed]
28. Delaney A, Angus DC, Bellomo R, et al. Bench-to-bedside review: the evaluation of complex interventions in critical care. Crit Care. 2008;12:210. [PMC free article] [PubMed]
29. Peake SL, Bailey M, Bellomo R, et al. Australasian resuscitation of sepsis evaluation (ARISE): A multi-centre, prospective, inception cohort study. Resuscitation. 2009;80:811–818. [PubMed]
30. Mansfield PF, Hohn DC, Fornage BD, et al. Complications and failures of subclavian-vein catheterization. N Engl J Med. 1994;331:1735–1738. [PubMed]
31. Sznajder JI, Zveibil FR, Bitterman H, et al. Central vein catheterization. Failure and complication rates by three percutaneous approaches. Arch Intern Med. 1986;146:259–261. [PubMed]
32. Eisen LA, Narasimhan M, Berger JS, et al. Mechanical complications of central venous catheters. J Intensive Care Med. 2006;21:40–46. [PubMed]
33. Lefrant JY, Muller L, De La Coussaye JE, et al. Risk factors of failure and immediate complication of subclavian vein catheterization in critically ill patients. Intensive Care Med. 2002;28:1036–1041.[PubMed]
34. Balls A, LoVecchio F, Kroeger A, et al. Ultrasound guidance for central venous catheter placement: results from the Central Line Emergency Access Registry Database. Am J Emerg Med. 2010;28:561–567. [PubMed]
35. Schummer W, Schummer C, Rose N, et al. Mechanical complications and malpositions of central venous cannulations by experienced operators. A prospective study of 1794 catheterizations in critically ill patients. Intensive Care Med. 2007;33:1055–1059. [PubMed]
36. Theodoro D, Krauss M, Kollef M, et al. Risk factors for acute adverse events during ultrasound-guided central venous cannulation in the emergency department. Acad Emerg Med. 2010;17:1055–1061. [PMC free article] [PubMed]
37. Bodhey NK, Gupta AK, Sreedhar R, et al. Retroperitoneal hematoma: an unusual complication after femoral vein cannulation. J Cardiothorac Vasc Anesth. 2006;20:859–861. [PubMed]
38. Akata T, Nakayama T, Kandabashi T, et al. Massive retroperitoneal hemorrhage associated with femoral vein cannulation. J Clin Anesth. 1998;10:321–326. [PubMed]
39. Durbec O, Viviand X, Potie F, et al. A prospective evaluation of the use of femoral venous catheters in critically ill adults. Crit Care Med. 1997;25:1986–1989. [PubMed]
40. Williams JF, Seneff MG, Friedman BC, et al. Use of femoral venous catheters in critically ill adults: prospective study. Crit Care Med. 1991;19:550–553. [PubMed]
41. Napolitano M, Malato A, Raffaele F, et al. Ultrasonography-guided central venous catheterisation in haematological patients with severe thrombocytopenia. Blood Transfus. 2013:1–5. doi: 10.2450/2013.0129-12. [PMC free article] [PubMed] [Cross Ref]
42. Carino GP, Tsapenko AV, Sweeney JD. Central line placement in patients with and without prophylactic plasma. J Crit Care. 2012;27:529.e9–e13. [PubMed]
43. Cavanna L, Civardi G, Vallisa D, et al. Ultrasound-guided central venous catheterization in cancer patients improves the success rate of cannulation and reduces mechanical complications: a prospective observational study of 1,978 consecutive catheterizations. World J Surg Oncol. 2010;8:91.[PMC free article] [PubMed]
44. Haas B, Chittams JL, Trerotola SO. Large-bore tunneled central venous catheter insertion in patients with coagulopathy. J Vasc Interv Radiol. 2010;21:212–217. [PubMed]
45. Della Vigna P, Monfardini L, Bonomo G, et al. Coagulation disorders in patients with cancer: nontunneled central venous catheter placement with US guidance–a single-institution retrospective analysis. Radiology. 2009;253:249–252. [PubMed]
46. Weigand K, Encke J, Meyer FJ, et al. Low levels of prothrombin time (INR) and platelets do not increase the risk of significant bleeding when placing central venous catheters. Med Klin (Munich)2009;104:331–335. [PubMed]
47. Tercan F, Ozkan U, Oguzkurt L. US-guided placement of central vein catheters in patients with disorders of hemostasis. Eur J Radiol. 2008;65:253–256. [PubMed]
48. Oguzkurt L, Tercan F, Kara G, et al. US-guided placement of temporary internal jugular vein catheters: immediate technical success and complications in normal and high-risk patients. Eur J Radiol. 2005;55:125–129. [PubMed]
49. Mumtaz H, Williams V, Hauer-Jensen M, et al. Central venous catheter placement in patients with disorders of hemostasis. Am J Surg. 2000;180:503–505. discussion 6. [PubMed]
50. Fisher NC, Mutimer DJ. Central venous cannulation in patients with liver disease and coagulopathy–a prospective audit. Intensive Care Med. 1999;25:481–485. [PubMed]
51. Doerfler ME, Kaufman B, Goldenberg AS. Central venous catheter placement in patients with disorders of hemostasis. Chest. 1996;110:185–188. [PubMed]
52. DeLoughery TG, Liebler JM, Simonds V, et al. Invasive line placement in critically ill patients: do hemostatic defects matter? Transfusion. 1996;36:827–831. [PubMed]
53. Foster PF, Moore LR, Sankary HN, et al. Central venous catheterization in patients with coagulopathy. Arch Surg. 1992;127:273–275. [PubMed]
54. Goldfarb G, Lebrec D. Percutaneous cannulation of the internal jugular vein in patients with coagulopathies: an experience based on 1,000 attempts. Anesthesiology. 1982;56:321–323. [PubMed]
55. Hall DP, Lone NI, Watson DM, et al. Factors associated with prophylactic plasma transfusion before vascular catheterization in non-bleeding critically ill adults with prolonged prothrombin time: a case-control study. Br J Anaesth. 2012;109:919–927. [PubMed]
56. Vinson DR, Hance LG, Mark DG, et al. for the KP CREST Network. Bleeding complications of central venous catheterization in septic patients with abnormal hemostasis. Ann Emerg Med. 2013;62:S134. [abstract 376]
57. Gross CP, Vogel EW, Dhond AJ, et al. Factors influencing physicians’ reported use of anticoagulation therapy in nonvalvular atrial fibrillation: a cross-sectional survey. Clin Ther. 2003;25:1750–1764.[PubMed]
58. Zikmund-Fisher BJ, Sarr B, Fagerlin A, et al. A matter of perspective: choosing for others differs from choosing for yourself in making treatment decisions. J Gen Intern Med. 2006;21:618–622.[PMC free article] [PubMed]
59. Spranca M, Minsk E, Baron J. Omission and commission in judgment and choice. J Exp Soc Psychol. 1991;27:76–105.