| Author | Affiliation |
|---|---|
| Jody L. Green, PhD | Rocky Mountain Poison and Drug Center, Denver Health Medical Center, University of Colorado, Denver, Colorado Vanderbilt University School of Nursing, Nashville, Tennessee |
| Kennon J. Heard, MD | Rocky Mountain Poison and Drug Center, Denver Health Medical Center, University of Colorado, Denver, Colorado University of Colorado, Department of Emergency Medicine, Aurora, Colorado |
| Kate M. Reynolds, MPH | Rocky Mountain Poison and Drug Center, Denver Health Medical Center, University of Colorado, Denver, Colorado |
| Donald Albert, MS | Rocky Mountain Poison and Drug Center, Denver Health Medical Center, University of Colorado, Denver, Colorado |
Introduction Methods Results Discussion Conclusion
ABSTRACT
Introduction: There are few reports summarizing the effectiveness of oral and intravenous (IV) acetylcysteine. We determined the proportion of acetaminophen poisoned patients who develop hepatotoxicity (serum transaminase > 1000 IU/L) when treated with oral and IV acetylcysteine.
Methods: Studies were double abstracted by trained researchers. We determined the proportions of patients who developed hepatotoxicity for each route using a random effects model. Studies were further stratified by early and late treatment.
Results: We screened 4,416 abstracts; 16 articles, including 5,164 patients, were included in the meta-analysis. The overall rate of hepatotoxicity for the oral and IV routes were 12.6% and 13.2%, respectively. Treatment delays are associated with a higher rate of hepatotoxicity.
Conclusion: Studies report similar rates of hepatotoxicity for oral and IV acetylcysteine, but direct comparisons are lacking. While it is difficult to disentangle the effects of dose and duration from route, our findings suggest that the rates of hepatotoxicity are similar for oral and IV administration.
INTRODUCTION
Acetaminophen poisoning is the most common medication poisoning reported to United States (U.S.) poison centers and accounts for more than 30,000 hospital admissions every year in the U.S. alone.1Fortunately, acetaminophen-related hepatotoxicity can be prevented by early treatment with acetylcysteine. Acetylcysteine is administered by either the intravenous (IV) route or the oral route. The use of IV acetylcysteine was studied in Europe and Australia in the early 1970s, while the use of oral acetylcysteine was studied in the U.S. in the late 1970s.2,3 Historically, IV acetylcysteine has been used in Canada, Europe, and Australia while oral acetylcysteine has been used in the U.S. In 2004, an IV formulation of acetylcysteine was approved for use in the U.S., and IV administration is now the most common route used in the U.S.4
As both IV and oral acetylcysteine are available in the U.S., clinicians must select one of these routes when treating an acetaminophen-poisoned patient. Unfortunately, there are no head-to-head trials comparing the efficacy of these 2 routes. The Cochrane Review of Interventions for Paracetamol (Acetaminophen) Overdose does not specifically compare IV and oral administration of acetylcysteine.5One previously published meta-analysis concluded that patients who present for treatment within 8 hours should be treated with IV acetylcysteine, but this analysis is now more than 10 years old and was performed before IV acetylcysteine was available in the U.S.6 More recently, Yarema et al7 compared the results of the U.S. National Multi-Center Trial of Acetylcysteine for Acetaminophen Overdose to the Canadian Acetaminophen Overdose Study. Their findings suggested that IV administration with a 21 hour administration protocol was more effective for patients presenting within 12 hours of ingestion and that oral administration with a 72 hour administration protocol was more effective for those presenting more than 18 hours after ingestion. These results have stimulated interest in systematically evaluating the efficacy of the oral and IV routes using all published data.
The objective of this study is to determine the percentage of patients who develop hepatotoxicity during treatment with oral and IV acetylcysteine, and to explore the time-dependence of efficacy for the 2 routes.
METHODS
This was a systematic literature review and meta-analysis of studies reporting patients treated with IV or oral acetylcysteine for acetaminophen overdose. The study protocol was not registered. For included studies, the primary outcome measures were the percentage of patients who developed hepatotoxicity during treatment acetylcysteine by either oral or IV administration. Our secondary aim was to evaluate the time-dependence of efficacy for each route.
Definitions
Hepatotoxicity was defined as a post-baseline aspartate aminotransferase (AST) or alanine aminotransferase (ALT) level above 1000 IU/L. The definition of a toxic acetaminophen concentration varied among the studies; most non-U.S. studies used the original definition of toxicity (concentration above the line starting at 200 mcg/ml at 4 hours) while U.S. studies used the modified definition (concentration above the line starting at 150 mcg/ml at 4 hours). We included studies using either definition.
Literature Search and Data Abstraction
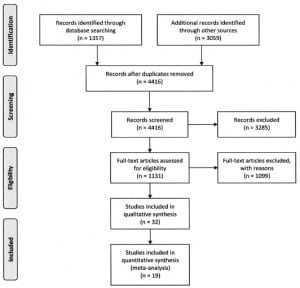
A literature search was performed using EMBASE, and MEDLINE and International Pharmaceutical Abstracts via Ovid. Articles were searched for acetaminophen key words using the terms acetaminophen, paracetamol, and CAS registry number 103-90-2, and for acetylcysteine key words using the terms acetylcysteine, n-acetylcysteine, and CAS registry number 616-91-1. The acetaminophen search was crossed with the acetylcysteine search using the Boolean operator “AND.” The literature search was limited to human exposure and English language articles published between 1966 and 2009. Article flow is presented in Figure 1. Manuscripts were also reviewed to identify and exclude duplicate reports of studies.
All abstracts from resultant citations were reviewed by a single reviewer to identify articles containing potential efficacy data. Full articles were obtained for all selected abstracts and were further reviewed by 2 researchers for inclusion. The inclusion criteria applied at this step were: use of acetylcysteine for acute acetaminophen overdose, use of acetylcysteine subsequent to a 4 to 24 hour acetaminophen level above the Rumack-Mathews treatment line (either the “150 line” or the “200 line”), no evidence of hepatotoxicity prior to acetylcysteine treatment, and a reported AST or ALT activity post acetylcysteine treatment.
All articles meeting these criteria were further abstracted by 2 trained researchers for demographic data (i.e. age, gender, race), study characteristics (i.e. study type and inclusion and exclusion criteria), route of treatment, and which line on the Rumack–Mathews nomogram was used to qualify patients for treatment. When reported, rates of hepatotoxicity were stratified by time to ingestion. Reconciliation of abstracted data was performed by a single researcher and disagreements were resolved by referencing the primary source. Abstractors were not blinded to the study objectives.
All full articles were reviewed for references of interest. Abstracts and citations of selected references were obtained and reviewed according to the literature search procedure. In addition, a cited reference search using Web of Science was performed on all articles selected for abstraction. Full articles reviewed during any step of the literature search process were also reviewed for potentially relevant references.
Final criteria used to determine inclusion of an article for analysis were: 1) acetylcysteine treatment, with route specified, 2) absence or presence of hepatotoxicity, and 3) a toxic acetaminophen concentration, defined using either the “150 line” or the “200 line” on the Rumack-Mathews nomogram. Other stratification parameters were time to initiation of acetylcysteine and study size. For the primary analysis, we included studies that had consecutive enrollment and at least 20 subjects, as these criteria were used by the Cochrane Review to decrease the effect of small studies with a high probability of selection bias.
Analysis Plan
As formal comparison of treatments using meta-analysis requires determining the differences in treatments using studies that directly compare treatments, we initially sought to identify studies that compared IV and oral administrations. As we did not find any studies meeting these criteria, we elected to present point estimates with 95% confidence intervals (CIs) for the outcomes of interest for each route rather than perform a formal metaanalysis comparing the routes.
Statistical Methods
The overall standardized estimates for event rates were determined for each route (IV or oral) and for time (early or late) subgroups. Event rates were defined as the number of subjects with post-baseline hepatotoxicity divided by the number of subjects included for a particular publication. Ninety-five percent confidence intervals were constructed on these estimates and compared across subgroups. Funnel plots were generated to investigate any publication bias. All meta-analyses, forest plots, and funnel plots were generated using “Comprehensive Meta- Analysis”® from Biostat™, Englewood, N.J., version 2.0.
The relationship between route (IV or oral) and time (early or late) of administration of NAC were explored using a multiple regression model. In order to construct estimable functions for route and time effects, we included only studies that reported both route and time. Time from acetaminophen ingestion to initiation of acetylcysteine therapy was abstracted as a binary outcome: “early” (less than 10 hours or as defined by the author) and “late” (greater than 10 hours or as defined by the author). A general linear mixed model (GLIMMIX procedure in SAS® version 9.2 Cary, N.C.) was applied to these data. Fixed effects were route, time, and the route by time interaction. Random effects were studied. Toxicity event rates were assumed to follow a binomial distribution. Main effects were considered significant if the P-value was < 0.05 and the interaction term was considered significant if the P-value was < 0.10. Standard meta-analysis techniques were used to summarize the data among the other citation subgroups. The meta-analysis was conducted using a random effects model.
RESULTS
The primary literature search identified 1357 citations of interest. An additional 3059 citations were identified by the Web of Science and selected reference searches. Together, 4416 abstracts were screened for data, with 1131 meeting necessary criteria for further review. Of the 1131 full articles reviewed for data, 334 were selected for abstraction. The results of the literature search are presented in Figure 1. After applying final inclusion criteria, 19 articles were eligible and included in 1 or more analyses. The vast majority of the 315 articles that did not meet inclusion criteria were excluded because they included less than 20 subjects. Other reasons for exclusion were because subjects were selected based on outcome, the route of NAC administration was not reported, and stratification, or outcome data were not reported in a way that allowed us to include the studies in the analysis.
Meta-analyses
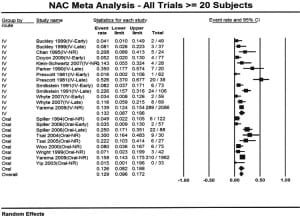
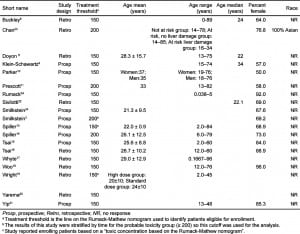
The characteristics of the patients included in the meta-analyses are shown in Table 1. Nineteen articles that met inclusion criteria and included at least 20 subjects were identified.3,6,8–24 Sixteen articles reporting 5164 unique cases were included in the overall meta-analysis.6,8–11,13–21,23–24 Three reports were excluded from this analysis as they were secondary data analyses or were included as a part of larger studies.3,22,25 The overall proportion of patients who developed hepatotoxicity in the studies meeting criteria for inclusion in the meta-analysis was 12.9% (95% confidence interval: 9.6% to 17.2%). The percentages were similar when studies were stratified by route (Figure 2); the proportion for IV treated patients was 13.2% (95% CI: 8.7% to 19.6%) while the proportion for oral treated patients was 12.6% (95% CI: 8.2% to 18.8%).
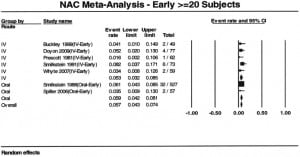
Seven reports provided outcome data stratified by early (n=949) and late (n=1293) treatment.3,6,10,11,14,17,24 Four studies used a 10 hour cutoff and 3 used an 8 hour cutoff. Patients who received early acetylcysteine therapy had a percentage of hepatotoxicity of 5.7% (95% CI: 4.3% to 7.4%), compared to 26% (95% CI: 23.6% to 29.0%) in the late acetylcysteine group (Figures 3 and and4).4). When the analysis was stratified by route and time to acetylcysteine, the proportion of hepatotoxicity for IV-early and oral-early were similar: 5.3% (95% CI: 3.2% to 8.5%) and 5.9% (95% CI: 4.2% to 8.1%), respectively. The percentages for the 2 routes were also similar for patients treated late: 23.3% (95% CI: 11.7% to 41.1%) for IV treatment and 26.3% (95% CI: 23.6% to 29.0%) for oral treatment.
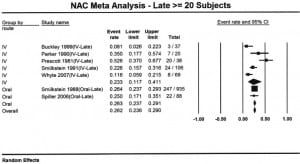
There was a marked difference in the percentage of patients who developed hepatotoxicity between early and late acetylcysteine administration. The regression analysis identified no significant route by time interaction (p=0.7516). However, there was a statistically significant effect due to time (p<0.001) and no significant effect due to route (p=0.7393). As a result of this analysis, it appears there is no difference in incidence of hepatotoxicity between acetylcysteine administered via IV or oral routes, but there is a difference between acetylcysteine administered early or late.
As we found a significant effect of time to treatment, the most relevant funnel plots to evaluate for publication bias must be stratified by route and time to treatment. This left only 2 studies in the oral group and 5 studies in the early/IV group, making formal analysis for publication bias impossible.
DISCUSSION
The percentage of patients who develop liver injury from acetaminophen poisoning is low for both oral and IV administration when acetylcysteine is administered early, generally defined as within 10 hours of ingestion. There is a marked increase in the percentage of subjects who develop hepatotoxicity when treatment is started more than 10 hours post ingestion, but the percentages for the oral and IV routes are similar in patients with delayed treatment. As the magnitude of the differences remained small, we feel the published literature reports similar rates of hepatotoxicity for the 2 routes.
The findings of our study are consistent with the findings of the previous meta-analysis, which reported an overall rate of hepatotoxicity with IV NAC of 3% with a 95% confidence interval of 0 to 6%, when treatment was initiated within 10 hours. Their results are based on 3 studies included in this meta-analysis. Our results include 2 additional studies with slightly higher rates of hepatotoxicity.
Our findings are also consistent with several studies that could not be included in the meta-analysis. Kerr performed a randomized controlled trial reporting 2 infusion rates of acetylcysteine. They noted no cases (0/58) of hepatotoxicity when acetylcysteine was infused within 8 hours and a hepatotoxicity rate of 9.8% (11/112) when treatment was delayed more than 8 hours after ingestion. Unfortunately, the study did not report how patients were risk stratified, so we were not able to include this study in our meta-analysis.26 Yarema et al7 reported a lower risk of hepatotoxicity for the IV route when treatment was initiated within 12 hours of ingestion, and the relative risk of hepatotoxicity for patients treated with IV at 10 hours (the cutoff used in our study) was approximately 0.7 compared to oral NAC. While we found that the point estimate of the percentage of patients who develop hepatotoxicity was lower for the patients treated early with IV NAC, the absolute difference was very small (less than 1%) and there was substantial overlap of the 95% confidence intervals for the estimates for each route. Unfortunately, Yarema et al7 evaluated time as a continuous rather than dichotomous variable, so we could not directly compare their findings to ours and were unable to include their full IV data in our comparison of time-stratified groups (a subset of the IV data was reported in another manuscript was included in our analysis).22 Furthermore, in the study by Yarema et al22 overall rate of hepatotoxicity (13.9%) was similar to our estimate (13.5%), suggesting that the populations were similar.
The efficacy of oral NAC using clinical (rather than time-based) endpoints has been described in several studies. Tsai et al16 described no cases of hepatotoxicity among 17 patients treated with oral NAC (140 mg/kg followed by 70 mg/kg every 4 hours for a minimum of 20 hours) and stopped once the acetaminophen was undetectable and the transaminases were normal. Using a similar protocol, Betten et al27–28 described no deaths and no cases of acute liver injury among 2137 patients. While the Betten et al27 studies could not be included in the meta-analysis because the laboratory testing was not reported in a manner to determine the presence or absence of hepatotoxicity (our primary outcome), the lack of clinical liver disease suggests that serious outcomes are unlikely if these endpoints are used. Many toxicologists now use some variation of this approach.
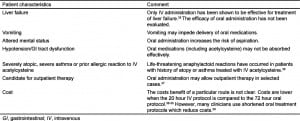
While we did not evaluate safety, a recently reported study compared the rates and frequency of adverse events for IV and oral administration of acetylcysteine.29 This study demonstrated that gastrointestinal effects (primarily nausea and vomiting) are common with both routes, but occur with a higher frequency with oral treatment, while anaphylactoid reactions were more common with IV administration. There were no acetylcysteine-related serious adverse events reported with either route. The authors concluded that the safety profile is acceptable for both routes. As our meta-analysis suggests that the efficacy of 21 hour IV and 72 hour oral acetylcysteine treatments are similar, we believe either route is acceptable depending on the patient’s circumstances. Another consideration is cost. Two studies have reported that costs are similar or slightly favor the 21 hour IV protocol over the 72 hour oral protocol. While there are several methodological limitations to these studies, they suggest that there is not a major difference in cost between the IV and oral route. Clinicians should select a route based on individual patient and institutional characteristics. We have listed several factors that clinicians should consider when determining the route for a particular patient (Table 2).
LIMITATIONS
There are limitations to any meta-analysis. The first limitation is that the studies included in the meta-analysis may be subject to publication bias. We identified a large number of studies, and our results suggest that there is little heterogeneity among the studies. In fact, the search results from this study are similar to the results reported in the Cochrane Review. Also, we may have missed some publications, as our search terms did not include the trade names (Acetadote, Fluimucil, Lysox, Mucolysin, Mucomyst, Parvolex) in the search terms.
A second limitation to any meta-analysis is that patient-level data is unavailable. Without patient level data, we could not account for several potential confounders that may be associated with outcome from acetaminophen poisoning. These confounders include age, sex, amount ingested, acute ethanol intoxication, chronic ethanol abuse, pre-existing liver disease, as well as the use of gastric decontamination and coingestions. It is also possible that there is residual confounding due to time from ingestion to treatment.
While our objective was to determine the rates of hepatotoxicity for the 2 routes, the optimal meta-analysis would compare oral and IV administration. However, we found no reports of trials with a direct comparison. An alternative to a head to head comparison would be to compare outcomes where each therapy was compared to placebo. However, there have been no placebo controlled trials of acetylcysteine, so this analysis was also impossible. Our analysis determined the overall rates reported for each route, but we did not perform a formal comparison of the rates.
Another limitation is that our analysis was focused only on route and did not account for dose. The large number of studies using different durations makes disentangling the effect of dose and duration from route in a meta-analysis very difficult. While we could have stratified by planned duration (i.e. 72 hour oral, 20 hour oral, 48 hour IV, 21 hour IV etc.), it is clear that even studies using “fixed” time points actually had variation in treatment duration. For example, patients treated with the “21 hour” IV protocol who develop hepatic injury will receive therapy beyond 21 hours and many of the studies evaluating oral administration used variable dosing duration rather than a fixed time.7,30–32
When the acetylcysteine treatment protocols are followed as approved, the oral treatment protocol provides approximately 5 times the amount of acetylcysteine as the IV treatment protocol over a 72 hour period. However, due to first pass effects, only a small percentage of the oral product is systemically bioavailable and systemic concentrations are likely substantially higher in the first 21 hours with IV dosing.33 The relative importance of hepatic and systemic concentrations is not known. As we observed very similar proportions of patients who developed hepatotoxicity for the oral and IV routes, we conclude that the effectiveness of published IV and oral protocols are similar.
A final limitation is that several of the identified studies did not report time to acetylcysteine in a way that allowed us to apply our early-late stratification scheme; therefore, several studies could not be included in the time-stratified analysis. This change in the data set produced point estimates of hepatotoxicity that were higher for IV administration (13.2% vs. 12.6%) in the overall analysis, but higher for oral administration in both early (5.9 vs. 5.3%) and late subgroups (26.3% vs. 23.3%). As the magnitude of the differences remained small, we feel our overall conclusions that the 2 routes have similar efficacy remain valid.
CONCLUSION
Studies report similar rates of hepatotoxicity for IV and oral acetylcysteine, but direct comparisons are lacking. Delays in treatment are associated with a dramatic increase in the rate of hepatotoxicity for both routes. While it is difficult to disentangle the effects of dose and duration from route, our findings suggest that the rates of hepatotoxicity are similar for oral and IV administration.
Footnotes
Supervising Section Editor: Brandon K. Wills, DO, MS
Submission history: Submitted September 1, 2011; Revisions received March 8, 2012; Accepted April 23, 2012
Full text available through open access at http://escholarship.org/uc/uciem_westjem
DOI: 10.5811/westjem.2012.4.6885
Address for Correspondence: Jody L. Green PhD, Denver Health and Hospital Authority, 660 Bannock Street, Denver, CO 80204.
Email: jody.green@rmpdc.org.
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Manthripragada AD, Zhou EH, Budnitz DS. Characterization of acetaminophen overdose-related emergency department visits and hospitalizations in the United States. Pharmacoepidemiol Drug Saf.2011;20(8):819–826. et al. [PubMed]
2. Prescott LF, Park J, Ballantyne A. Treatment of paracetamol (acetaminophen) poisoning with N-acetylcysteine. Lancet. 1977;2(8035):432–434. et al. [PubMed]
3. Smilkstein MJ, Knapp GL, Kulig KW. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985) N Eng J Med.1988;319(24):1557–1562. et al. [PubMed]
4. Bronstein AC, Spyker DA, Cantilena LR. 2009 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 27th Annual Report. Clin Toxicol (Phila)2010;48(10):979–1178. et al. [PubMed]
5. Brok J, Buckley N, Gluud C. Interventions for paracetamol (acetaminophen) overdose. Cochrane Database Syst Rev. 2006;2006(2):CD003328. [PubMed]
6. Buckley NA, Whyte IM, O’Connell DL. Oral or intravenous N-acetylcysteine: Which is the treatment of choice for acetaminophen (paracetamol) poisoning? J Toxicol Clin Toxicol. 1999;37(6):759–767. et al. [PubMed]
7. Yarema MC, Johnson DW, Berlin RJ. Comparison of the 20-hour intravenous and 72-hour oral acetylcysteine protocols for the treatment of acute acetaminophen poisoning. Ann Emerg Med.2009;54(4):606–614. et al. [PubMed]
8. Doyon S, Klein-Schwartz W. Hepatotoxicity despite early administration of intravenous N-acetylcysteine for acute acetaminophen overdose. Acad Emerg Med. 2009 2009;16(1):34–39. [PubMed]
9. Klein-Schwartz W, Doyon S. Acetylcysteine for early presenting overdoses of acetaminophen combination products. Clin Toxicol. 2007;45:346.
10. Parker D, White JP, Paton D. Safety of late acetylcysteine treatment in paracetamol poisoning. Hum Exp Toxicol. 1990;9(1):25–27. et al. [PubMed]
11. Prescott LF. Treatment of severe acetaminophen poisoning with intravenous acetylcysteine. Arch Intern Med. 1981;141(3 Spec No):386–389. [PubMed]
12. Rumack BH, Peterson RC, Koch GG. Acetaminophen overdose. 662 cases with evaluation of oral acetylcysteine treatment. Arch Intern Med. 1981;141(3 Spec No):380–385. et al. [PubMed]
13. Spiller HA, Krenzelok EP, Grande GA. A prospective evaluation of the effect of activated charcoal before oral N-acetylcysteine in acetaminophen overdose. Ann Emerg Med. 1994;23(3):519–523. et al.[PubMed]
14. Spiller HA, Winter ML, Klein-Schwartz W. Efficacy of activated charcoal administered more than four hours after acetaminophen overdose. J Emerg Med. 2006;30(1):1–5. et al. [PubMed]
15. Tsai CL, Chang WT, Weng TI. Acute acetaminophen intoxication in Taiwan: outcomes and risk factors. J Formos Med Assoc. 2004;103(11):830–835. et al. [PubMed]
16. Tsai C-L, Chang W-T, Weng T-I. A patient-tailored N-acetylcysteine protocol for acute acetaminophen intoxication. Clin. Ther. 2005;27(3):336–341. et al. [PubMed]
17. Whyte IM, Francis B, Dawson AH. Safety and efficacy of intravenous N-acetylcysteine for acetaminophen overdose: analysis of the Hunter Area Toxicology Service (HATS) database. Curr Med Res Opin. 2007;23(10):2359–2368. [PubMed]
18. Woo OF, Mueller PD, Olson KR. Shorter duration of oral N-acetylcysteine therapy for acute acetaminophen overdose. Ann. Emerg. Med. 2000 2000;35(4):363–368. et al. [PubMed]
19. Wright RO, Anderson AC, Lesko SL. Effect of metoclopramide dose on preventing emesis after oral administration of N-acetylcysteine for acetaminophen overdose. J Toxicol Clin Toxicol. 1999;37(1):35–42. et al. [PubMed]
20. Yarema MC, Johnson DW, Berlin RJ. Comparison of the 20- Hour Intravenous and 72-Hour Oral Acetylcysteine Protocols for the Treatment of Acute Acetaminophen Poisoning. Ann Emerg Med.2009;54(4):606–614. et al. [PubMed]
21. Yip L, Dart RC. A 20-hour treatment for acute acetaminophen overdose. N Eng J Med.2003;348(24):2471–2472. [PubMed]
22. Sivilotti ML, Yarema MC, Juurlink DN. A risk quantification instrument for acute acetaminophen overdose patients treated with N-acetylcysteine. Ann Emerg Med. 2005;46(3):263–271. et al. [PubMed]
23. Chan TYK, Critchley JAJH, Chan AYW. Renal failure is uncommon in Chinese patients with paracetamol (Acetaminophen) poisoning. Vet Hum Toxicol. 1995;37(2):154–156. [PubMed]
24. Smilkstein MJ, Bronstein AC, Linden C. Acetaminophen overdose: a 48-hour intravenous N-acetylcysteine treatment protocol. Ann Emerg Med. 1991;20(10):1058–1063. et al. [PubMed]
25. Rumack BH, Peterson RG. Acetaminophen overdose: incidence, diagnosis, and management in 416 patients. Pediatrics. 1978;62(5 Pt 2 Suppl):898–903. [PubMed]
26. Kerr F, Dawson A, Whyte IM. The Australasian clinical toxicology investigators collaboration randomized trial of different loading infusion rates of N-acetylcysteine. Ann Emerg Med.2005;45(4):402–408. et al. [PubMed]
27. Betten DP, Cantrell FL, Thomas SC. A prospective evaluation of shortened course oral N-acetylcysteine for the treatment of acute acetaminophen poisoning. Ann Emerg Med. 2007;50(3):272–279. et al. [PubMed]
28. Betten DP, Burner EE, Thomas SC. A retrospective evaluation of shortened-duration oral N-acetylcysteine for the treatment of acetaminophen poisoning. J Med Toxicol. 2009;5(4):183–190. et al.[PMC free article] [PubMed]
29. Bebarta VS, Kao L, Froberg B. A multicenter comparison of the safety of oral versus intravenous acetylcysteine for treatment of acetaminophen overdose. Clin Toxicol (Phila) 2010;48(5):424–430. et al. [PMC free article] [PubMed]
30. Kerr F, Dawson A, Whyte IM. The Australasian Clinical Toxicology Investigators Collaboration randomized trial of different loading infusion rates of N-acetylcysteine. Ann Emerg Med.2005;45(4):402–408. et al. [PubMed]
31. Betten DP, Cantrell FL, Thomas SC. A prospective evaluation of shortened course oral N-acetylcysteine for the treatment of acute acetaminophen poisoning. Ann Emerg Med. 2007;50(3):272–279. et al. [PubMed]
32. Woo OF, Mueller PD, Olson KR. Shorter duration of oral N-acetylcysteine therapy for acute acetaminophen overdose. Ann Emerg Med. 2000;35(4):363–368. et al. [PubMed]
33. Borgstrom L, Kagedal B, Paulsen O. Pharmacokinetics of N-acetylcysteine in man. Eur J Clin Pharmacol. 1986;31(2):217–222. [PubMed]
34. Rumack BH. Acetaminophen overdose in young children. Treatment and effects of alcohol and other additional ingestants in 417 cases. Am J Dis Child. 1984;138(5):428–433. [PubMed]
35. Harrison PM, Keays R, Bray GP. Improved outcome of paracetamol-induced fulminant hepatic failure by late administration of acetylcysteine. Lancet. 30 1990;335(8705):1572–1573. et al. [PubMed]
36. Appelboam AV, Dargan PI, Knighton J. Fatal anaphylactoid reaction to N-acetylcysteine: caution in patients with asthma. Emerg Med J. 2002;19(6):594–595. [PMC free article] [PubMed]
37. Heard KJ. Acetylcysteine for acetaminophen poisoning. N Engl J Med. 2008;359(3):285–292.[PMC free article] [PubMed]
38. Marchetti A, Rossiter R. Managing acute acetaminophen poisoning with oral versus intravenous N-acetylcysteine: a provider-perspective cost analysis. J Med Econ. 2009;12(4):384–391. [PubMed]
39. Martello JL, Pummer TL, Krenzelok EP. Cost Minimization Analysis Comparing Enteral N-Acetylcysteine to Intravenous Acetylcysteine in the Management of Acute Acetaminophen Toxicity. Clin Toxicol (Phila) 2010;48(1):79–83. [PubMed]


