| Author | Affiliation |
| Richard M. Nowak, MD | Henry Ford Health System, Department of Emergency Medicine, Detroit, Michigan |
| Prabath Nanayakkara, MD | VU University Medical Center, Department of Emergency Medicine, Amsterdam, Netherlands |
| Salvatore DiSomma, MD | Sant’ Andrea Hospital, Department of Emergency Medicine, Rome, Italy |
| Phillip Levy, MD | Wayne State University, Department of Emergency Medicine and Cardiovascular Research Institute, Detroit, Michigan |
| Edmée Schrijver, MD | VU University Medical Center, Department of Emergency Medicine, Amsterdam, Netherlands |
| Rebecca Huyghe, RN | Henry Ford Health System, Department of Emergency Medicine, Detroit, Michigan |
| Alessandro Autunno, MD | Sant’ Andrea Hospital, Department of Emergency Medicine, Rome, Italy |
| Robert L. Sherwin MD | Wayne State University, Department of Emergency Medicine and Cardiovascular Research Institute, Detroit, Michigan |
| George Divine, PhD | Henry Ford Health System, Department of Biostatistics, Detroit, Michigan |
| Michele Moyer, BSN | Henry Ford Health System, Department of Emergency Medicine, Detroit, Michigan |
Introduction
Methods
Results
Discussion
Limitations
Conclusion
ABSTRACT
Introduction
Noninvasive hemodynamic (HD) assessments in the emergency department (ED) might assist in the diagnosis, therapeutic plan development and risk stratification of acutely ill patients. This multinational observational study was designed to initiate noninvasive HD measurements prior to any ED patient therapeutic interventions and broadly evaluate them for potential diagnostic, therapeutic and predictive value.
Methods
We enrolled patients with suspected acute heart failure (AHF), sepsis or stroke. Continuous noninvasive HD monitoring was begun using the Nexfin finger cuff device (Edwards LifeSciences, BMEYE, Amsterdam, Netherlands). Beat-to-beat HD measurements were averaged for the initial 15 minutes, prior to therapeutic intervention. We performed suspected disease group comparisons and evaluated HD predictors of 30-day mortality.
Results
Of 510 patients enrolled: 185 (36%) AHF, 194 (38%) sepsis and 131 (26%) stroke. HD variables were significantly different (p<0.05) amongst the groups. Cardiac output and index and stroke volume index (SVI) were highest in sepsis (6.5, 3.5, 36), followed by stroke (5.5, 2.7, 35.8), and lowest in AHF (5.4, 2.7, 33.6). The in-group HD standard deviations and ranges measurements were large, indicating heterogeneous underlying HD profiles. Presenting SVI predicted 30-day mortality for all groups.
Conclusion
Presenting ED noninvasive HD data has not been previously reported in any large patient population. Our data suggest a potential role for early noninvasive HD assessments aiding in diagnosing of patients, individualizing therapy based on each person’s unique HD values and predicting 30-day mortality. Further studies and analyses are needed to determine how HD assessments should be best used in the ED.
INTRODUCTION
Acutely ill patients present daily to the emergency department (ED) requiring evaluation and treatment. To help distinguish amongst patients with similar symptoms, emergency physicians (EPs) use historical information, initial/repeat vital signs and physical examinations and results of laboratory, electrocardiographic, radiologic and ultrasonographic testing to estimate underlying hemodynamic (HD) status. Such estimates are important for making time-critical diagnoses, formulating therapeutic resuscitation plans and risk stratifying patients for disposition.
However, multiple studies report that physician estimations of HD values are inconsistent and inaccurate when compared to objective measurements. This has been reported for cardiologists,1 intensivists,2 trauma surgeons,3,4 and Eps.5,6 Thus, it is likely that acutely ill ED patients have suboptimal HD assessments. This increases the potential for inadequate/inappropriate management, unsuitable dispositions, and potentially avoidable adverse events.
Established technologies used to objectively measure HD variables have not been integrated into ED care as they require invasive methods; are time consuming to apply and calibrate; considered unreliable; can only be used intermittently; or a combination of these factors. Noninvasive measures of the HD status of ED patients are being increasingly used, adding new dimensions to monitoring variables.7 However much of these applications are still being researched, and there have been no large studies reporting objectively measured pretreatment HD values of acutely ill patients.
We designed the Prognostic Hemodynamic Profiling in the Acutely Ill Emergency Department Patient (PREMIUM) multinational registry, a large prospective observational study using a novel monitoring device (Nexfin, Edwards LifeSciences, BMEYE, Amsterdam, Netherlands) to noninvasively and continuously measure the initial two to four hours of beat-to-beat HD measurements in a large number of ED patients with clinically suspected acute heart failure (AHF), sepsis or stroke. The presenting ED 15-minute averaged HD assessments are reported for the first time in these patient groupings and are broadly evaluated for potential diagnostic, therapeutic or predictive value.
METHODS
This was a prospective, observational, study of acutely ill patients presenting to the ED with EP suspicion of AHF, sepsis or stroke. Suspected disease states were studied as the investigators wished to capture presenting patient hemodynamics, unaltered by any ED therapy, before recording any further HD changes throughout the monitoring period. When available a trained clinical research associate obtained informed consent and then applied the Nexfin device using a standardized, previously reported methodology.8 Continuous noninvasive finger cuff derived HD measurements were recorded for 2 – 4 hours. If a patient was required to leave the ED for diagnostic testing the Nexfin recording was paused until the patient returned, when it was restarted.
Treating EPS and nurses were blinded to all HD monitoring (Nexfin screen covered). Patient baseline characteristics, including reported race and medical history/medications were recorded. Also ED initial vital signs, testing and therapies, final diagnosis, length of stay (LOS), and disposition along with all in-hospital testing and therapies, total hospital LOS, final discharge diagnosis and discharge location were documented on standardized case report forms (CRFs). Patient-reported discomfort with finger cuff monitoring was also noted. Mortality and unscheduled medical visits were tracked (by phone and/or medical record review) through 30 days post-discharge.
Patients were enrolled in the PREMIUM registry from September 2010 – September 2012 in four large urban academic medical centers: Henry Ford Health System, Detroit, Michigan, USA (coordinating center); Detroit Receiving Hospital, Wayne State University School of Medicine, Detroit, Michigan, USA; VU University Medical Center, Amsterdam, Netherlands; and Sant’Andrea Hospital, University La Sapienza, Rome, Italy. The two U.S. medical centers were university affiliated (annual ED visits of 94,000 for each) while the two European sites were university hospitals (annual ED volumes of 32,000 and 55,000 respectively). The study was registered prior to being initiated. (ClinicalTrials: gov number; NCT01208077)
Individuals 18 years old who after initial evaluation by EPs were thought to have AHF, sepsis or stroke and had received no prior HD-altering therapy (supplemental oxygen and IV fluids at <50 ml/hour were allowed) were eligible for inclusion. If an ED patient required any additional therapy on presentation and before possible enrollment they were not eligible. The study investigators chose these three clinical conditions as a reasonable mix of common, acute medical problems that would yield a heterogeneous collection of HD measurements.
Suspected AHF patients were required to have as their primary complaint recurrent or worsening shortness of breath (< 3 days) thought by the EP to be caused by heart failure, a natriuretic peptide (BNP, NT pro BNP, MR-pro ANP) measurement ordered and a prior established heart failure diagnosis. Suspected sepsis individuals needed to have acute symptoms and signs (< 3 days), as clinically assessed by the EP, thought to be due to sepsis and blood cultures and/or blood lactate measurement planned. Suspected stroke patients had abnormal neurologic symptoms/signs (< 24 hours) believed by the EP to be caused by stroke and a non-contrast head computed tomography ordered.
Patients were excluded if they were unable to provide informed consent, could not be enrolled within four hours of ED arrival, had end stage renal disease (ESRD) (requiring hemo or peritoneal dialysis), were suspected or confirmed to be pregnant, had acute ST-segment elevation myocardial infarction (STEMI), unavailable for 30-day follow up, had any Do Not Resuscitate status, known to have aortic valvular disease, transferred from another facility, excessively agitated, were receiving any ongoing intravenous home infusions, had a left ventricular assist device (LVAD), were previously enrolled in the PREMIUM registry or were currently in a therapeutic investigational study. ESRD patients were excluded as their HD profiles could have been dialysis dependent; those with aortic valvular disease as the Nexfin HD measurements are not accurate in the presence of aortic insufficiency, agitated patients, as excessive hand motion makes it difficult to keep the finger cuff in proper position, and those with an LVAD as they have non pulsatile blood flow.
This study was conducted in accordance with the ethical principles of the Declaration of Helsinki and the International Conference on Harmonization Guidance for Good Clinical Practice. The protocol was approved by each participating center’s institutional review board or ethics committee and written or oral informed consent was obtained from each subject before enrollment.
Nexfin is a FDA-approved and CE-marked (European Union cleared) device that uses pulse contour analysis to derive digital artery blood pressure (BP) noninvasively and continuously through proprietary finger cuff technology, based on the volume clamp method developed by Penaz and Wesseling.9,10 Cardiac output (CO) and other HD variables are calculated from a reconstructed brachial artery BP waveform using the Nexfin CO-Trel pulse contour method.11,12 The systemic vascular resistance (SVR) is calculated (mean BP – central venous pressure [CVP]) x 80/CO) with CVP fixed at 5 mm Hg. While the CO, SVR and other HD variables are beat-to-beat screen displayed in real time, measurements are also recorded enabling trending over time. When the peripheral finger arteries are severely constricted (significant circulatory shock, patients receiving vasopressors or who are hypothermic etc.) HD measuring may become very difficult or even impossible, and in this case the Nexfin issues a warning that no plethysmogram is detected.
All patient Nexfin recordings were inspected by BMEYE (blinded to any patient clinical data) to insure that they were analyzable. If the tracings in any individual patient were not thought to be generally analyzable, the principle author (RMN) reviewed the tracings, and if in agreement the subject was removed from the registry. The beat-to-beat measurements showed some variations/artifacts (Figure 1) as subjects were not intubated or sedated, could move freely and interact with relatives and ED personnel. To smooth out the Nexfin recordings we averaged all beat-to-beat measurements for each recorded minute. With any break in the monitoring, five complete minute averages prior to and post stoppage were averaged together and this value imputed for each minute average during the stoppage. This was done so that future trend analyses could compare patient values at the same real-time point after initiation of monitoring. For this report the presenting HD measurements are the averages of the first 15 complete minute averages recorded.
We performed statistical analysis using SAS 9.2 (SAS Institute Inc., Cary, NC, SAS/STAT 9.2 Users Guide, 2008) with level of significance set at p ≤ 0.05. We used Chi-square tests to compare proportions and ANOVA to compare the mean continuous variables between the suspected disease specific groups. Logistic regression modeling was used to determine 30-day mortality predictors. To assess the predictive ability of each model we computed the c-statistic or area under the receiver-operator characteristic curve (AUC). All statistical analyses were completed by Henry Ford Health System statisticians.
As the PREMIUM Registry was strictly an observational trial recording the presenting underlying HD profiles of acutely ill ED patients (never studied before) no power calculations were completed for enrollment numbers needed to determine a difference in the HD measurements between the three disease states studied.
Figure 1. Nexfin continuous hemodynamic recording. Nexfin continuous HD recording in a non-sedated, non-intubated patient showing beat to beat variability. Averaging the first 15 minutes (shaded area) smooths out these variations.
RESULTS
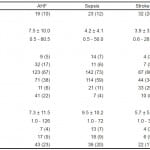
We enrolled 514 subjects in the study. Four (0.8%) were excluded (2 AHF, 2 sepsis) as their Nexfin recordings were not analyzable, thought to be secondary to finger cuff failure. There were no PREMIUM enrolled patients for whom the Nexfin device was unable to provide continuous noninvasive HD monitoring even though some had prior documented peripheral vascular disease. Less than 5% of patients expressed some discomfort in the monitored finger and this was resolved by moving the finger cuff to an alternate digit. Thus, the final registry consisted of 510 patients: 185 (36%) AHF, 194 (38%) sepsis and 131 (26%) stroke. Suspected group baseline demographic and clinical characteristics are shown in Table 1.
Table 1. Baseline characteristics of patients studied.
AHF, acute heart failure; COPD; chronic obstructive pulmonary disease
Overall preexisting comorbidities were common but within each suspected disease grouping the relative proportions differed significantly. Coronary artery disease (29%), congestive heart failure (100%), chronic obstructive lung disease (40%), diabetes (52%), myocardial infarction (34%), hypertension (81%), renal disease (40%), cardiac pacemaker (18%) and peripheral vascular disease (9%) were more frequently encountered in the AHF patients, while cancer (29%) and infections (28%) were more reported in those with sepsis. Cerebrovascular disease (18%) and smoking history (27%) were most often found in stroke patients.
Patient group LOS, disposition and outcome significantly differed in the suspected disease groupings (p-values, Table 2). Stroke patients had the highest ED discharge rate (24%), followed by sepsis (11%) with AHF subjects the lowest (10%). The mean ED LOS (hours) was longest in the AHF group (7.5), followed by sepsis (4.2) and shortest in stroke (3.9). Sepsis subjects were admitted to a general ward most often (59%), followed by AHF (38%) and stroke the least (34%). Stroke patients were most admitted to an intensive care unit (ICU), usually a stroke unit, (25%) with sepsis next (11%) and AHF the least (6%). AHF patients were most often transferred to an observation unit (17%) and admitted to telemetry (22%).
Table 2. Patient disposition, length of stay (LOS) and outcomes.
AHF, acute heart failure; ED, emergency department; LOS, length of stay; ICU, intensive care unit
Admitted mean LOS (days) was for sepsis 9.5, 7.3 for AHF and 5.7 for stoke with large ranges. Group rates of in hospital mortality, 30-day death and unscheduled medical visits post discharge were not significantly different: AFH, 4%, 9%, 23%; sepsis, 7%, 9%, 20%; and stroke 3%, 5% , 17% respectively.
The 15-minute averaged mean HD variables were significantly different in the suspected disease groupings (p-values, Table 3). Blood pressures (systolic, diastolic, and mean in mm Hg) were highest in stroke and lowest in sepsis with AHF in between Heart rates (beats per minute) were highest in sepsis (96.6), followed by AHF (83.0) and lowest in stroke (77.6). Measurements of cardiac function (cardiac output, cardiac index [CI] and stroke volume index [SVI]) were highest in sepsis (6.5, 3.5, 36), followed by stroke (5.5, 2.7, 35.8), and lowest in AHF (5.4, 2.7, 33.6). The SVR was highest in stroke, followed by AHF and lowest in sepsis (1787.6, 1483.7, 1172.1 respectively). The within suspected group standard deviations and ranges for HD values were large, indicating very diverse HD measurements in patient populations with the same suspected disease.
Table 3. Presenting 15-minute averaged hemodynamic parameters.
AHF, acute heart failure; BP, blood pressure; SVR, systemic vascular resistance; SVRI, systemic vascular resistance index
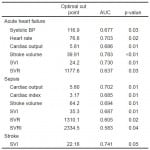
Univariate HD parameters associated with 30-day mortality on logistic regression are reported in Table 4. AHF and sepsis had six individual predictors each and stroke had one. The single common 30-day mortality predictor for all groups was SVI (Figure 2) with different optimal cohort cut points (AHF 24.2, sepsis 35.3, stroke 22.16). Of note systolic blood pressure (SBP) and HR were only predictive of 30-day mortality for AHF.
Table 4. Significant hemodynamic predictors for 30-day mortality.
ROC, receiver operator characteristic curve; AUC, area under curve; BP, blood pressure; SVI, stroke volume index; SVR, systemic vascular resistance; SVRI, systemic vascular resistance index
Figure 2. Stroke volume index receiver operating curves for 30-day mortality.
A, acute heart failure; B, sepsis; C, stroke; AUC, area under curve
DISCUSSION
Overall the multinational patient population studied was quite ill, having numerous medical comorbidities with relatively high rates of hospitalization, long LOS and high rates of 30-day unscheduled medical visits and mortality. No clinical scales or scoring systems were used to further define the severity of disease in the three studied groups. This patient population would benefit from enhanced ED assessments and resulting therapies to improve clinical outcomes.
The presenting HD variables differed significantly amongst patients with suspected AHF, sepsis and stroke. These differences potentially could be useful to help distinguish one disease state from another, especially when overlapping features, such as dyspnea, are present. For example, without HD knowledge patients might be misdiagnosed with volume overload AHF and treated with diuretics when in fact they have infection with cardiac dysfunction due to high output failure. Disease differentiation using HD studies may be less important in those with suspected stroke syndromes.
However, most importantly, within each of the three patient groupings widely diverse HD profiles were seen indicating that individuals with suspected similar diseases have broadly different HD characteristics that are clinically unrecognized by treating physicians. Knowing an individual patient’s presenting HD measurements enables increased understanding of their cardiovascular function and also may provide potential targets for optimal treatment. Early predictors of in-hospital mortality for AHF individuals are reported to be a BUN of > 43 mg/dl, serum creatinine of > 2.75 mg/dl and a systolic BP < 115 mmHg.13 Others recommend scoring systems combining multiple clinical variables to risk stratify AHF patients.14 However, these recommendations provide no specific guidance for therapeutic actions whereas the HD measurements might direct interventions. Additionally, modalities like the echocardiographic-determined ejection fraction (EF) do not help in patient management as they correlate poorly to CO (providing only crude measure of left ventricular contraction) without insight into SVR.15
If given optimal therapy guided by HD monitoring it might be possible to drive down hospital LOS and 30-day readmission rates (25%) in this patient population.16 However, additional analyses of our data and further studies are needed to determine how to best manipulate these HD variables. It is known that patients with worsening heart failure in the first week of admission have low cardiac power and higher SVR.17 These individuals could benefit by very early vasodilator therapy that is titrated by SVR monitoring as this results in significantly improved symptoms and renal function in those patients with inadequate response to standard therapies.18 Patients with poor forward flow as evidenced by persistently low CO/CI/SVI might benefit from admission to an ICU where HD-guided therapy and management by heart failure specialists could occur. AHF patients with high CI/SVI would have additional therapy directed to the cause of the high output failure (sepsis, anemia etc.) rather simply aiming for volume reduction.
In septic patients the Mortality in Emergency Department Sepsis (MEDS) score, the Confusion, Urea nitrogen, Respiratory rate, Blood pressure, 65 years of age and older (CURB-65) score and a modified Rapid Emergency Medicine Score (mREMS) predict 28-day hospital mortality.19 However, as with AHF, this information does not give direction for therapy. Nearly one in four admitted patients with sepsis, who lack evidence of shock or end-organ dysfunction on presentation, progress within 72 hours to severe sepsis or shock and those who develop septic shock have higher 30-day mortality than those who do not (8% v 2%).20 These individuals are typically thought initially to be hemodynamically stable in the ED, based mostly on BP and pulse values, and are thus are admitted to a non-monitored/ICU setting. ED sepsis patients at risk for disease progression might be identified by noninvasive HD monitoring (low CI) and treated more aggressively. The ability to identify cardiac dysfunction (low CI) may be particularly valuable as this perturbation is common in patients admitted with sepsis and septic shock and serves as a major predictor of in hospital mortality.21
Furthermore, patients with community-acquired pneumonia who experience disease progression and have late (1-9 days post admission) transfer to the ICU have higher 30-day mortality than those transferred within 24 hours of admission (47% v 23%).22 Septic patients meeting criteria for early goal-directed therapy with low ED CI have higher in-hospital mortality.23 This is secondary to reduced cardiac contractility requiring larger fluid boluses or early adjunctive inotropes to achieve resuscitation goals.24 Thus, early HD monitoring of ED sepsis patients and prompt identification and further treatment of specific patients with low CI could potentially alter their outcomes.
Current early prognostic HD assessment of acute ischemic stroke involves BP. Elevated SBP on arrival is common (>184 mm Hg in 15%) but tends to decrease spontaneously within 90 minutes of stroke onset.25 Some report a U-shaped relationship between admission BP and better outcomes (optimal SBP 121-200, diastolic blood pressure [DBP] 81-110mmHg)26 while others do not, making best BP management unclear. Existing guidelines recommend that stroke patients not be given thrombolytic medications when the SBP >220 or DBP >120 mmHg.27 As we have shown, there is heterogeneity in the presenting BP and other HD parameters in those suspected of stroke, and SVI, not BP, was associated with 30-day mortality. Thus knowledge of HD values may supersede the value of BP alone. This is not surprising as BP correlates poorly with CI.28 Stroke patients admitted to an ICU overall have high SVRI and decreased CI and SVI, while non-surviving ischemic stroke patients have significantly even higher mean SVRI compared to survivors.29 Additionally, rehabilitating stroke patients with a low EF (<35%) and presumed lower CI are less likely to return home.30 Maintaining cerebral blood flow to the ischemic penumbra of the brain is important. However, in the 2013 AHA/ASA Stroke guidelines27 there is no mention of HD assessment of these patients beyond BP. Further studies are needed to more completely characterize the meaning of HD variables in acute stroke and how they might be manipulated to improve outcomes.
LIMITATIONS
There are limitations to this report. This observational study was of convenience design and thus possibly not a representative sample of patients with the suspected diseases studied. Most but not all patients had their suspected disease verified upon further ED testing or during hospitalization. Additional analyses will be needed and are planned comparing the HD variables amongst those with confirmed to those with suspected disease. While we report individual HD variables as predictors of 30-day mortality, significant overlap exists for these variables and it will be necessary to distill out the ones that are most relevant. We have not yet analyzed the trending of HD variables over time, their response to treatments or what specific values were seen at monitoring end. These additional results could alter the predictive values of the variables reported. The study was carried out in urban academic medical centers and so measurements in other settings might be different.
CONCLUSION
In summary, technological advances now allow for routine noninvasive continuous monitoring of HD variables in acutely ill ED patients. We have reported for the first time the presenting ED HD values of large patient cohorts with suspected AHF, sepsis and stroke as measured by the Nexfin device. These initial HD measurements may be important for improving diagnosis, developing individualized therapeutic management plans/disposition decisions and predicting 30-day mortality. Further clinical studies are needed to determine how to best use these now obtainable HD measurements in the ED.
Footnotes
Supervising Section Editor: John Bedolla, MD
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Address for Correspondence: Richard M. Nowak, MD, Henry Ford Health System, Emergency Department, 2799 West Grand Boulevard, Detroit, Michigan, 48202. Email: rnowak1@hfhs.org.
Submission history: Submitted January 23, 2014; Accepted August 21, 2014
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
- Stevenson LW, Perloff JK. The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. JAMA. 1989;261:884-88.
- Eisenberg PR, Jaffe AS, Schuster DB. Clinical evaluation compared to pulmonary artery catheterization in the hemodynamic assessment of critically ill patients. Crit Care Med. 1984;12:549-53.
- Lopez-Saucedo A, Hirt M, Appel PL, et al. Feasibility of noninvasive physiologic monitoring in resuscitation of trauma patients in the emergency department. Crit Care Med. 1989;17:567-68.
- Veale WN, Morgan JH, Beatty JS, et al. Hemodynamic and pulmonary fluid status in the trauma patient: are we slipping? Am Surg. 2005;71:621-26.
- Neath SX, Lazio L, Guss DA. Utility of impedance cardiography to improve physician estimation of hemodynamic parameters in the emergency department. Congest Heart Fail. 2005;11:17-20.
- Nowak RM, Sen A, Garcia AJ et al. The inability of emergency physicians to adequately clinically estimate the underlying hemodynamic profiles of acutely ill patients. Am J Emerg Med. 2012;30:954-60.
- Middleton PM, Davis SR. Noninvasive hemodynamic monitoring in the Emergency Department. Curr Opin Crit Care. 2011;17:342-50.
- Nowak RM, Sen A, Garcia AJ et al. Noninvasive continuous or intermittent blood pressure and heart rate monitoring in the Emergency Department. Amer J Emerg Med. 2011;29:782-89.
- Wesseling KH. A century of noninvasive arterial pressure measurement: from Marey to Penaz and Finapres. Homeostasis. 1995;36:2-3.
- Wesseling KH, De Wit B, Vander Hoeven GMA, et al. Physiocal, calibrating finger vascular physiology for Finapres. Homeostasis. 1995;36:67-82.
- Girdulich P, Prentza A, Wesseling KH. Models of brachial to finger pulse wave distortion and pressure decrement. Cardiovasc Res. 1997;33:698-705.
- Bogert LW, Wesseling KH, Schera O, et al. Pulse contour cardiac output derived from non-invasive arterial pressure in cardiovascular disease. Anesthesia. 2010;65:119-25.
- Adams KF, Uddin N, Patterson JH. Clinical predictors of in-hospital mortality in acutely decompensated heart failure – Piecing together the outcome puzzle. Cong heart Fail. 2008;14:127-34.
- Stiell IG, Clement CM, Brison RJ, et al. A risk scoring system to identify Emergency Department patients with heart failure at high risk for serious adverse events. Acad Emerg Med. 2013;20:17-26.
- Uriel N, Torre-Amione G, Milo O, et al. Echocardiographic ejection fraction in patients with acute heart failure: correlations with hemodynamic, clinical, and neurohormonal measures and short term outcomes. Eur J Heart Fail. 2005;7:815-19.
- Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnosis and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355-63.
- Torre-Amione G, Milo-Cotter O, Kaluski E, et al. Early worsening heart failure in patients admitted for acute heart failure: Time course, hemodynamic predictors, and outcome. J Cardiac Fail. 2009;15:639-44.
- Frea S, Franco E, Najd K, at al. Refractory acute decompensated heart failure: an observational study on a noninvasive hemodynamic monitoring system aimed at improving the therapeutic approach. J Cardiovasc Med. 2012;11:655-61.
- Howell MD, Donnino MW, Talmor D, et al. Performance of severity of illness scoring systems in emergency department patients with infection. Acad Emerg Med. 2007;14:709-14.
- Glickman SW, Cairns CB, Otero R, et al. Disease progression in hemodynamically stable patients presenting to the emergency department with sepsis. Acad Emerg Med. 2012;17:383-90.
- Landesberg G, Gilon D, Meroz Y, et al. Diastolic dysfunction and mortality in severe sepsis and septic shock. Eur Heart J. 2012;33:895-903.
- Restrepo MI, Mortensen EM, Rello J, et al. Late admission to the ICU in patients with community-acquired pneumonia is associated with higher mortality. Chest. 2010;137:552-57.
- Napoli AM, Machan JT, Corl K, et al. The use of impedance cardiography in predicting mortality in Emergency Department patients with severe sepsis and septic shock. Acad Emerg Med. 2010;17:452-55.
- Napoli AM, Corl K, Gardiner F, et al. Prognostic value of noninvasive measures of contractility in Emergency Department patients with severe sepsis and septic shock undergoing early goal-directed therapy. J Crit Care. 2011;26:47-53.
- Quershi AI, Ezzeddine MA, Nasar A, et al. Prevalence of elevated blood pressure in 563,704 adult patients with stroke presenting to the ED in the United States. Amer J Emerg Med. 2007;25:32-38.
- Vemmos KN, Tsivgoulis G, Spengos K, et al. U-shaped relationship between mortality and admission blood pressure in patients with acute stroke. J Intern Med. 2004;255:257-65.
- Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: A guideline for health care professionals form the American Heart Association/American Stroke Association. Stroke. 2013.
- Wo C, Shoemaker W, Appel P, et al. Unreliability of blood pressure and heart rate to evaluate output in emergency resuscitation and critical illness. Crit Care Med. 1993;21:218-23.
- Ramirez MFL, Tibayan RT, Marinas CE, et al. Prognostic value of hemodynamic findings from impedance cardiograph in hypertensive acute stroke. Amer J Hypertens. 2005;18:65S-72S.
- Kevorkian SG, Nambiar SV, Rintala DH. Low ejection fraction: Effect on rehabilitation progress and outcomes of stroke patients. Amer J Phys Med Rehabil. 2005;84:655-61.