| Author | Affiliation |
|---|---|
| Jan M. Shoenberger, MD | Keck School of Medicine of the University of Southern California, Department of Emergency Medicine, Los Angeles, CA |
| Serineh Voskanian, MD | Keck School of Medicine of the University of Southern California, Department of Emergency Medicine, Los Angeles, CA |
| Sara Johnson, BS | Keck School of Medicine of the University of Southern California, Los Angeles, CA |
| Terence Ahern, BA | Keck School of Medicine of the University of Southern California, Los Angeles, CA |
| Sean O. Henderson, MD | Keck School of Medicine of the University of Southern California, Department of Emergency Medicine, Los Angeles, CA Keck School of Medicine of the University of Southern California, Department of Preventive Medicine, Los Angeles, CA |
ABSTRACT
Introduction:
While research has established that the bedside electrocardiogram (ECG) is an insensitive test for the presence or absence of left ventricular hypertrophy (LVH), the finding, when present, is thought to be reproducible. To assess the reproducibility of serial ECGs done in the emergency department (ED) with regard to the presence or absence of LVH.
Methods:
A prospective study on consecutive patients admitted to an ED-run cardiac observation unit. A single reviewer collected and scored ECGs for the presence of LVH, using three established criteria (Cornell, Sokolow-Lyon and Romhilt-Estes). Demographic and medical history was also collected.
Results:
Over a three-year time period, 295 patients were enrolled; 132 males and 163 females with a mean age of 54.4 years (range, 19–89 years). The prevalence of LVH ranged from 11–14% and the agreement among all three criteria was fair (kappa = 0.325). Using the Cornell criteria, 33 patients had ECG#1 consistent with LVH. Of the patients meeting LVH criteria on ECG #1, only 15 retained their diagnosis of LVH on ECG#2 (i.e. 55% of the LVH identified in ECG#1 was not seen in ECG#2). Additionally, nine patients developed an ECG diagnosis of LVH between ECG#1 and ECG#2. In total, 27 (nine percent of the total) had ECG measurements that changed between ECG#1 and ECG#2. We made similar findings with the Sokolow-Lyon and Romhilt-Estes criteria. The results were not modified by gender, blood pressure or medication use.
Conclusion:
The finding of LVH on ECG was not very reproducible during serial measurements on the same person during a single 24-hour observation period.
INTRODUCTION
The presence of left ventricular hypertrophy (LVH) has been reported to carry significant cardiovascular risk.1–5 Although echocardiography and cardiac MRI are superior to electrocardiogram (ECG) for the diagnosis of LVH, these modalities are not readily available in the emergency department (ED).6–9 Instead, emergency physicians (EP) rely on ECG when risk stratifying a patient who presents with acute chest pain. The need to use this rapid bedside test to make the diagnosis of LVH has led to the development of multiple tools to interpret LVH on ECG, e.g. Cornell voltage, Sokolow-Lyon and Romhilt-Estes criterion. The specificities of these tools are high (>90%), but the sensitivities are low (20–60%).10–14 In at least one large retrospective study, the overall sensitivity of ECG diagnosis of LVH was found to be 6.9%.15 In addition, the findings of LVH on ECG may resolve over time. A number of studies have described patients diagnosed with LVH using ECG technology only to show significant regression with appropriate anti-hypertensive treatment during subsequent years.16–20
If EPs are to utilize the finding of LVH on ECGs to risk stratify individuals presenting with acute chest pain, the test should be reproducible during the course of a single ED visit. Previous authors have demonstrated that a single ECG is not a sensitive measure of LVH, but there is no published data on the reproducibility of serial ECGs in the acute setting. We undertook a prospective study using ED patients presenting with chest pain to examine the reproducibility of serial ECGs to identify LVH.
METHODS
Study Population
This was a convenience sample of patients presenting to a large, urban ED with a chief complaint of chest pain. Patients were included in the study if they had a minimum of two electrocardiograms performed to rule out a myocardial infarction in the ED and/or the adjacent ED-run chest pain unit (CPU). Participants were excluded if their electrocardiograms were unreadable due to poor technique or if the patient had bundle branch or atrio-ventricular block on ECG.
Study Design
Data was gathered prospectively as patients were admitted to the departmental CPU. We collected demographic information, clinical data and copies of standard 12-lead electrocardiograms during the ED/CPU stay. Vital signs and pertinent medications, specifically the use of digitalis, were abstracted from the medical charts. A single EP independently read and interpreted each ECG for the presence or absence of LVH using (a) the Cornell voltage criteria, (b) the Sokolow-Lyon voltage criteria, and (c) the Romhilt-Estes score. According to the Cornell voltage criteria, LVH is present if the sum of RaVLand SV3 is greater than or equal to 28 mm in men and 20 mm in women. The Sokolow-Lyon voltage criteria identify LVH when either RaVL is greater than 1.1mV or the sum of SV1 and RV5 or RV6 is greater than or equal to 3.5 mV. The Romhilt-Estes point score system determines LVH to be probable for four points and definite for five or more points; however, we combined those individuals with Romhilt-Estes scores of four and five into an LVH (+) classification. Previous authors have used this threshold of ≥four to ease interpretation and assess the true prevalence and cardiovascular risk associated with LVH.21 The EP interpreting the electrocardiograms was blinded to all data except gender and the use of digitalis.
Statistical Analysis
We performed statistical analysis using STATA 9.0 software (College Station, TX), and generated a Kappa statistic to test the agreement between the Cornell voltage criteria, Sokolow-Lyon criteria, and the Romhilt-Estes score. We then used logistic regression analysis to study the associations between change in criteria determination from ECG #1 to ECG #2 and age, race, gender and the change in mean arterial pressure from ECG #1 to ECG #2.
RESULTS
Between December 2004 and May 2007, 295 patients were included in the study; 132 males and 163 females with a mean age of 54.4 years (range, 19–89 years) (Table 1). Most of the patients were Latinos (65%) and ranged from 50–69 years old (60%).
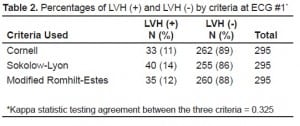
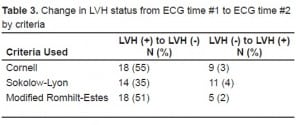
Table 2 details the prevalence of LVH in the population for each of the three criteria used. Overall, the prevalence ranged from 11–14% in our patient population. The agreement between the three criteria was fair (kappa = 0.325). When using the Cornell criteria, 33 patients (11%) tested positive for LVH on ECG#1 and 262 had no evidence of LVH on ECG#1. Only 15 of 33 (45%) who tested positive for LVH on ECG#1 retained their original diagnosis on ECG#2. Furthermore, nine patients out of 262 (3%) who tested negative for LVH during ECG#1 subsequently tested positive during ECG#2. In total, 27 patients (9% of the total) had ECG measurements that changed between ECG#1 and ECG#2. We noted similar findings with all three criteria used, although neither the Sokolow-Lyon criteria nor the modified Romhilt-Estes criteria demonstrated such a dramatic change (Table 3).
To explain the variation seen in ECG criteria for LVH over time, we controlled for the mean arterial pressure (MAP) during analysis; however, we saw no consistent effect (data not shown). Age, gender and ethnicity also had no effect on our findings.
DISCUSSION
When evaluating an ED patient with chest pain, EPs risk-stratify patients to estimate probability of a diagnosis of acute coronary syndrome. One data point in this decision is the 12-lead ECG. Since LVH is known to be an independent predictor of future cardiovascular events and all-cause mortality, its identification on ECG implies increased risk to the patient, thus necessitating a more extensive patient work-up. Unfortunately, this logic has been challenged by recent research that suggests ECG technology provides an insensitive marker for the presence or absence of LVH.10–14 It also appears that there is a large degree of variability from ECG to ECG within the same individual. Three previous reports describe the variability of electrocardiographic diagnosis of LVH.22–24 These studies demonstrate inconsistent amplitude and duration of P waves, QRS complexes and ST and T wave measurements in certain leads during ECGs taken minutes to 24 hours apart on the same person. If ECG is used to risk stratify an individual with possible acute coronary syndrome, then it is important that the test be reproducible.
The presence of LVH has been shown to predict a higher rate of future cardiovascular events compared to those patients without LVH. In a sample of men, De Bacquer et al.1(using Sokolow-Lyon) demonstrated that ECG diagnosed LVH was significantly associated with cardiovascular disease death (RR = 3.14). In a different multicenter study of patients presenting to the ED with symptoms of acute coronary syndrome, Pope et al.3 (using Cornell voltage) found that patients with ECG-LVH were six times as likely to have a confirmed diagnosis of congestive heart disease and three times as likely to have hypertension. In addition, they discovered the 30-day mortality among patients with ECG-LVH was 4.6%. Mansoor et al.25 (using Sokolow-Lyon) describe rates of hypertension complications, which include stroke, hypertensive heart failure and myocardial infarction, retinopathy, and aortic aneurysm, to be two to four times higher in patients with ECG-LVH.25 This effect has been demonstrated in multiple populations including patients with renal disease, the elderly and those with coronary artery disease.4,7,26
The presence of LVH has also been considered a marker of sustained hemodynamic and neurohormonal stress on the myocardium.4 In the Health Outcomes Prevention Evaluation trial (using Sokolow-Lyon), LVH was present in 8.3% of a high-risk population undergoing treatment with ACE inhibitors. Patients had a single ECG performed at the time of randomization that was read by the local site investigator. For those patients with ECG measurements demonstrating evidence of LVH, the relative risk (RR) of sustaining a major CV event was 1.3 compared to those patients without LVH and the RR of all-cause death was 1.53.4
In our study of patients presenting to an ED with acute chest pain, diagnostic changes occurred in the interpretation of ECG#1 versus ECG#2 regarding LVH in approximately nine percent of patients; thus, the test was reproducible in 91% of the total patient population. However, in those patients that tested positive for LVH in ECG#1 using Cornell criteria, only 45% retained findings of LVH on ECG#2. Similarly, three percent of patients who tested negative for LVH in ECG#1 subsequently tested positive after ECG#2. We noted similar findings using Sokolow-Lyon criteria and the modified Romhilt-Estes criteria. While we did not investigate the reason for these changes, potential variables include situational stress, transient or untreated hypertension, lead placement, or underlying heart disease.
As mentioned, one possible explanation for the misdiagnosis of LVH in our patient population is lead placement. Angeli et al.23 demonstrated the profound effect that lead placement can have on the presence of LVH by repeating electrocardiograms on hypertensive patients within 24 hours. Compared to our study, they found a similar proportion of patients changed their classification of LVH from the first to the second ECG. In 1990, Farb et al.24 demonstrated a high variability in the measurement of LVH when serial ECG measurements were separated by eight days. Although the lead to lead variability was high in that study, only two to three percent of individuals were reclassified as having LVH or not. Therefore, one approach to minimize the type of variability would be to leave the leads in place during the entire ED/CPU visit.
LIMITATIONS
A primary limitation of this study was that we did not confirm the presence of LVH with an echocardiogram in real time; so we have no gold standard against which to judge the accuracy of the ECG in making the diagnosis of LVH. In addition, the ECGs were analyzed by one EP to limit the confusion associated with multiple reviewers; however, this introduced a possible rater bias. Lastly, the ECG leads were placed by different emergency ECG technicians, and the time period between ECGs varied during the 24-hour observation period.
CONCLUSION
Our study demonstrates that patients presenting with acute chest pain to the ED often have ECG findings of LVH that are not reproducible. Therefore, the utility of diagnosing LVH by ECG in patients with acute chest pain is yet to be determined.
Footnotes
Supervising Section Editor: Tareg Bey, MD
Submission history: Submitted August 09, 2007; Revision Received November 08, 2008; Accepted December 03, 2008.
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Address for Correspondence: Jan M. Shoenberger, MD, Department of Emergency Medicine, LAC + USC Medical Center, 1200 N. State Street Room 1011, Los Angeles, CA 90033
Email: sohender@usc.edu
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. De Bacquer D, De Backer G, Kornitzer M, et al. Prognostic value of ECG findings for total, cardiovascular disease, and coronary heart disease death in men and women. Heart.1998;80:570–7. [PMC free article] [PubMed]
2. Kahn S, Frishman WH, Weissman S, et al. Left ventricular hypertrophy on electrocardiogram: prognostic implications from a 10-year cohort study of older subjects: a report from the Bronx Longitudinal Aging Study. J Am Geriatr Soc. 1996;44:524–9.[PubMed]
3. Pope JH, Ruthazer R, Kontos MC, et al. The impact of electrocardiographic left ventricular hypertrophy and bundle branch block on the triage and outcome of ED patients with a suspected acute coronary syndrome: a multicenter study. Am J Emerg Med. 2004;22:156–63. [PubMed]
4. Lonn E, Mathew J, Pogue J, et al. Relationship of electrocardiographic left ventricular hypertrophy to mortality and cardiovascular morbidity in high-risk patients. Eur J Cardiovasc Prev Rehabil. 2003;10:420–8. [PubMed]
5. Savonitto S, Ardissino D, Granger CB, et al. Prognostic value of the admission electrocardiogram in acute coronary syndromes. JAMA. 1999;281:707–13. [PubMed]
6. Alfakih K, Walters K, Jones T, et al. New gender-specific partition values for ECG criteria of left ventricular hypertrophy: recalibration against cardiac MRI. Hypertension.2004;44:175–9. [PubMed]
7. Sundström J, Lind L, Arnlöv J, Zethelius B, et al. Echocardiographic and electrocardiographic diagnoses of left ventricular hypertrophy predict mortality independently of each other in a population of elderly men. Circulation. 2001;103:2346–51. [PubMed]
8. Walsh TF, Hundley WG. Assessment of ventricular function with cardiovascular magnetic resonance. Cardiol Clin. 2007;25:15–33. [PubMed]
9. Crow RS, Prineas RJ, Rautaharju P, et al. Relation between electrocardiography and echocardiography for left ventricular mass in mild systemic hypertension. Am J Cardiol.1995;75:1233–8. [PubMed]
10. Verdecchia P, Schillaci G, Borgioni C, et al. Prognostic value of a new electrocardiographic method for diagnosis of left ventricular hypertrophy in essential hypertension. J Am Coll Cardiol. 1998;31:383–90. [PubMed]
11. Verdecchia P, Angeli F, Reboldi G, et al. Improved cardiovascular risk stratification by a simple ECG index in hypertension. Am J Hypertens. 2003;16:646–52. [PubMed]
12. Gasperin CA, Germiniani H, Facin CR, et al. An analysis of electrocardiographic criteria for determining left ventricular hypertrophy. Arq Bras Cardiol. 2002;78:72–83.
13. Pewsner D, Juni P, Egger M, et al. Accuracy of electrocardiography in diagnosis of left ventricular hypertrophy in arterial hypertension: systematic review. BMJ.2007;335:711–4. [PMC free article] [PubMed]
14. Vottonen P, Husso M, Sipola P, et al. Electrocardiographic left ventricular hypertrophy has low diagnostic accuracy in middle-aged subjects. Blood Press.2007;16:328–34. [PubMed]
15. Levy D, Labib SB, Anderson KM, et al. Determinants of sensitivity and specificity of electrocardiographic criteria for left ventricular hypertrophy. Circulation.1990;81:815–820. [PubMed]
16. Lindholm LH, Ibsen H, Dahlof B, et al. Cardiovascular morbidity and mortality in patients with diabetes in the losartan intervention for endpoint reduction in hypertension study (LIFE): a randomized trial against atenolol. Lancet. 2002;359:1004–10. [PubMed]
17. Schlaich MP, Schmieder RE. Left ventricular hypertrophy and its regression: pathophysiology and therapeutic approach. Am J Hypertens. 1998;11:1394–1404.[PubMed]
18. Mathew J, Sleight P, Lonn E, et al. Reduction of cardiovascular risk by regression of electrocardiographic markers of left ventricular hypertrophy by the angiotensin-converting enzyme inhibitor ramipril. Circulation. 2001;104:1615–21. [PubMed]
19. Okin PM, Devereux RB, Jern S, et al. Regression of electrocardiographic left ventricular hypertrophy by losartan versus atenolol: the losartan intervention for endpoint reduction in hypertension (LIFE) study. Circulation. 2001;108:684–90.[PubMed]
20. Okin PM, D Devereux RB, Jern S, et al. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA. 2004;292:2343–9. [PubMed]
21. Pewsner D, Jüni P, Egger M, et al. Accuracy of electrocardiography in diagnosis of left ventricular hypertrophy in arterial hypertension: systemic review. BMJ.2007;335(7622):711. [PMC free article] [PubMed]
22. Van Den Hoogen JP, Mol WH, Kowsoleea A, et al. Reproducibility of electrocardiographic criteria for left ventricular hypertrophy in hypertensive patients in general practice. Eur Heart J. 1992;13:1606–10. [PubMed]
23. Angeli F, Verdecchia P, Angeli E, et al. Day-to-day variability of electrocardiographic diagnosis of left ventricular hypertrophy in hypertensive patients. Influence of electrode placement. J Cardiovasc Med (Hagerstown) 2006;7:812–6. [PubMed]
24. Farb A, Devereux RB, Kligfeld P. Day-to-day variability of voltage measurements used in electrocardiographic criteria for left ventricular hypertrophy. J Am Coll Cardiol.1990;15:618–23. [PubMed]
25. Mansoor GA, Massie BM. Left ventricular hypertrophy: a potent cardiovascular risk factor and its relationship to office and ambulatory blood pressure. Blood Press Monit.1999;4(supp 1):S19–22. [PubMed]
26. Rigatto C, Foley R, Jeffery J, et al. Electrocardiographic Left ventricular hypertrophy in renal transplant recipients: prognostic value and impact of blood pressure and anemia.J Am Soc Nephrol. 2003;14:462–8. [PubMed]


