| Author | Affiliation |
|---|---|
| Robert Jones, MD | Madigan Army Medical Center, Department of Emergency Medicine, Tacoma, WA |
| Brandon Wills, DO | Madigan Army Medical Center, Department of Emergency Medicine, Tacoma, WA |
| Christopher Kang, MD | Madigan Army Medical Center, Department of Emergency Medicine, Tacoma, WA |
ABSTRACT
Chlorine gas represents a hazardous material threat from industrial accidents and as a terrorist weapon. This review will summarize recent events involving chlorine disasters and its use by terrorists, discuss pre-hospital considerations and suggest strategies for the initial management for acute chlorine exposure events.
INTRODUCTION
Once used as a chemical weapon in the World War I, chlorine gas has long been known for its pulmonary irritant properties. Many chlorine exposures are the result of accidents at swimming pools and mixing of household agents. However, recent events in the Iraq war, the war on terror and large-scale industrial incidents have demonstrated that chlorine represents a persistent hazardous material (HAZMAT) threat. Massive quantities of chlorine are stored and transported across the United States with minimal security. Chlorine has been successfully used as an unconventional weapon in Operation Iraqi Freedom. In this article, we will review the domestic threats of chlorine exposure, discuss its use as an unconventional weapon, review toxicologic principles, and examine treatment of chlorine-related casualties. We will also discuss some pertinent disaster management aspects of chlorine-related mass casualty (MASCAL) incidents.
Industrial production of chlorine in the U.S. exceeds 15 million tons annually. It is used in numerous industrial practices, including the manufacturing of paper, plastic and chemical products. It is also widely used in the municipal treatment of sewage and drinking water. Since chlorine production occurs in fewer than 20 states, it must be transported in large quantities to metropolitan centers. This large-scale transport and storage close to urban centers could make it an attractive target for extremist organizations. The use of chlorine could allow terrorists to deploy a chemical weapon in a highly populated area without having to manufacture or transport it themselves.1 An intentional release of chlorine by terrorists, bombing of storage vessels, or an unintentional release from an industrial incident could produce thousands of casualties. In fact an intentional release of chlorine, included as one of the Department of Homeland Security’s 15 “National Planning Scenarios,” could result in over 17,000 fatalities and 100,000 injuries if it occurred in a highly populated area2,3. Despite this, many at-risk communities are unaware of the volume of chlorine that is transported through their area. The following events document recent incidents involving chlorine releases and highlight some of the risks posed by chlorine gas.
RECENT INCIDENTS
January 6, 2005, Graniteville, SC
One of the worst chlorine accidents in recent years occurred in Graniteville, South Carolina. A misplaced track switch resulted in a collision between a railroad tanker carrying chlorine and another train. The chlorine tank ruptured, venting 90 tons of chlorine gas into the surrounding area. The release resulted in nine fatalities. Eight were due to asphyxiation and occurred immediately. The ninth occurred within a day of admission to the hospital. There were also over 520 visits to local emergency departments (EDs) or primary care offices for-chlorine related symptoms.4 Other non-medical effects included disruption of the local community, with over 5,400 of the town’s 7,000 residents being evacuated for several days.4,5
February 12, 2007, Tacoma, WA
Due to technician error when transferring chlorine from a rail car to storage containers in a bleach factory, over 900 pounds of chlorine gas were released, forcing the closure of the Port of Tacoma.6,7 Although there were no fatalities, 25 people required medical attention, including 12 first responders who were overcome after the wind shifted, placing them directly in the path of the chlorine gas. Despite the relatively low number of casualties caused by this incident, closure of North America’s seventh largest container port illustrates how a chlorine leak causes major disruption of transportation and shipping industries.
August 29, 2007, Las Vegas, NV
Operator error caused a Union Pacific tanker car full of chlorine gas to accidentally escape from the Arden train yard. Although no toxic gas was released, the tanker traveled 20 miles through densely populated areas before railroad workers and police were able to stop it. Based on prior estimates by the University of Nevada, the release of a full tanker of chlorine in such a populated area could have caused up to 90,000 fatalities.8,9
Operation Iraqi Freedom, 2007
Insurgents in Iraq have recently executed multiple attacks using chlorine tanker trucks. On February 20, 2007, a tanker truck carrying chlorine was outfitted with explosives and detonated in Taji. Five people were killed in the blast and over 140 others sought medical treatment for chlorine exposure.10,11 The next day, another attack with chlorine resulted in two fatalities and over 30 treated at nearby hospitals for respiratory complaints.12 On March 16, 2007, insurgents conducted three separate attacks using chlorine. The attacks occurred during a three-hour period in several different cities. Over 350 civilian casualties resulted from exposure, with hundreds requiring medical treatment.13 On April 6, 2007, another incident involving a chlorine truck primed with explosives left 30 dead with over 50 seeking care at local hospitals for respiratory symptoms.14 Most of these attacks utilized explosive-fit chlorine tanker trucks. These unorthodox delivery methods demonstrate a willingness by extremist groups to use chlorine in heavily populated areas, and highlight the relative ease in acquiring large quantities. Weaponized chlorine has clearly been shown to result in significant morbidity, mortality, and societal impact.
CLINICAL CONSIDERATIONS
General Toxicology
Chlorine is element number 17 on the periodic table. It exists as a yellow-green gas at temperatures above −34°C. Because this gas has a density greater than air, it tends to settle along the ground.15 All irritant gases have the potential to cause pulmonary injury, which is principally related to the duration of exposure. Irritant gases with high water solubility (e.g. ammonia) interact with oral, nasal and ocular mucosa rapidly causing discomfort. This warning property allows people to leave an area of exposure, limiting contact and thus reduces the likelihood of significant pulmonary damage. Irritant gases with low water solubility (e.g. phosgene) generate damaging caustic and oxidizing byproducts more slowly, resulting in very little warning properties. The absence of robust warning properties may result in prolonged exposures, allowing these agents to reach distal airways and produce greater pulmonary injury. Chlorine gas has an intermediate solubility, which gives it some warning properties, but may also allow for prolonged exposure and pulmonary damage.16,17 At 1-3 parts per million (PPM), chlorine starts to cause irritation of the mucus membranes. Pulmonary symptoms begin at exposures greater than 15 PPM, and concentrations greater than 430 PPM are fatal within 30 minutes (Table 1).18,19 In comparison, the Occupational Safety and Health Administration (OSHA) has set the permissible exposure limit (PEL) for an eight-hour time period at 1 PPM.20

Pathophysiology
Chlorine’s toxic effects are due primarily to the production of hypochlorous and hydrochloric acid that occurs when elemental chlorine (Cl2) reacts with water. These two acids then further react to produce oxygen free radicals (Figure 1). In large exposures, the acids and free radicals then damage cell walls and interact with sulfhydryl groups on various amino acids and enzyme systems.16,18Histologic findings of this damage include bronchial edema, desquamation of epithelial cells, erosions and localized necrosis.2,21
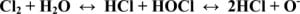
Clinical Effects
Local irritation from acid results in an inflammatory response of the upper and lower airways leading to bronchospasm, cough and dyspnea. Disruption of cell membranes and proteins by the acids and free radicals results in death of alveolar cells and endothelial cells of adjacent capillaries. Clinical manifestations of this damage include pulmonary edema and Acute Respiratory Distress Syndrome (ARDS).16,22 Fortunately, most patients with mild to moderate exposures will have resolution of their acute symptoms within three to five days and will have normal pulmonary function tests after several months.15,23 Some patients, however, will have chronic respiratory problems, such as reactive airway disease (RAD). While there is no way to predict which patients will develop long term complications, patients with a history of smoking or a prior history of RAD may be at increased risk..18
Although the most serious clinical effects of chlorine toxicity tend to be pulmonary, it can also cause skin and eye injuries. While dermal manifestations include irritation and pain, in severe exposures, chemical burns, including blister formation, can take place. Ocular complications include irritation and conjunctivitis. Severe cases can result in corneal defects.24 Chlorine also causes other non-specific symptoms, such as nausea, vomiting, rhinorrhea and headache.25
MANAGEMENT STRATEGIES OF CHLORINE EXPOSURE
Pre-hospital and Personal Protective Measures
The most important aspect of managing a chlorine release is identification of the prevailing wind direction in order to stage upwind while establishing scene safety. Personal protective equipment (PPE) for all first responders is paramount. The incident in Tacoma resulted in pre-hospital provider injuries due to limited utilization of PPE and an unexpected shift in wind direction. This event illustrates the importance of the use of appropriate PPE for all responders, which should include self-contained respirators as well as eye and skin protection.19 According to OSHA standards, all personnel moving into high-risk areas must be issued positive pressure ventilation systems.26 This level of protection best corresponds to OSHA level A or level B PPE since both consist of positive pressure self-contained breathing apparatuses with full face plates as well as protective over garments.26 Per OSHA standard 910.120, if this type of system is not initially used, those conducting the preliminary assessment must be issued an escape breathing apparatus capable of operation for at least five minutes.26 Military personnel working in a contaminated area or working on the decontamination line should be outfitted in mission-oriented protective posture (MOPP) level 4 equipment.27 MOPP level 4 is roughly equivalent to OSHA level C PPE because it uses a chemical protective mask instead of a positive pressure breathing system. While this level of protection is insufficient to enter areas of high concentration, such as near a leaking chlorine tank, it does provide adequate protection for perimeter duty and work on the decontamination line. MOPP level 4 also represents the highest level of PPE that all U.S. soldiers are trained to use. Experience with higher levels of PPE, such as positive pressure respiratory systems, is reserved for specialized military HAZMAT teams.
Decontamination
Treatment of patients in the pre-hospital setting consists principally of decontamination and supporting the patient’s breathing. Decontamination, as with most chemical exposures, involves evacuation of the patient from the area of exposure, removing clothing, and irrigating with copious amounts of water. Although the duration of decontamination has not been conclusively identified, three to five minutes of rinse time has been advocated.19 While 0.5% chlorine solution is recommended for the decontamination of undifferentiated chemical exposures,27 it is not expected to be relevant in cases of chlorine-related casualties. Patients complaining of ocular irritation should undergo copious eye irrigation. Contact lenses should be removed to ensure proper irrigation.15,16Unlike people exposed to liquid chlorine, patients exposed to only chlorine vapor represent no significant risk of contaminating rescue workers (“off gasing”).24 Patients exposed to gaseous chlorine probably will not require any decontamination other than removal of the clothing. However, if they are experiencing ocular or dermal symptoms, they should undergo decontamination.2
Treatment
Pre-hospital support of respiratory injuries involves removing patients from the source of exposure, providing supplemental oxygen, and administering inhaled beta-agonists for patients with bronchospasm. For patients in extreme respiratory distress, rapid sequence intubation (RSI) should be considered, but this type of resource-intensive procedure may not be feasible if there are multiple casualties and limited resources cannot support such an intervention. The use of a standardized triage system can help to sort patients based on the severity of their injuries and to guide resource allocation in such a situation.
ED care of patients is supportive. However, it is very important that the ED be prepared to decontaminate patients who arrive via private vehicles since these patients may not have been decontaminated prior to arrival. During the disaster in Graniteville, over 95% of the patients arrived via private vehicle.4 After appropriate decontamination, skin wounds should be treated like other chemical burns, including: irrigation, tetanus prophylaxis, local wound care and analgesia. Eye irritation should prompt copious lavage and evaluation for corneal defects. Severe eye injuries, including any that result in a corneal defect, require ophthalmology consultation. If ophthalmologic consultation is not immediately available, patients with corneal defects should receive topical antibiotic prophylaxis to prevent bacterial superinfection. These patients also require ophthalmologic follow-up within the next few days.
Mild respiratory symptoms and bronchospasm can be treated with aerosolized beta-agonists. If a patient has a persistent cough but no respiratory distress, nebulized lidocaine (4 mL of a 4% solution) may provide symptomatic relief.28 For patients with severe respiratory distress, RSI should be considered. Patients may develop ARDS, with its characteristic bilateral infiltrates on chest x-ray and hypoxia that does not improve with increased inspired oxygen concentration. Therefore, when a patient is intubated after a chlorine exposure, low tidal volumes (5–8 mL/kg ideal body weight) are recommended.29 The patient’s inspired oxygen concentration and peak expiratory end pressure (PEEP) can then be adjusted to maintain the patient’s oxygen saturation.
There is no specific antidote for chlorine exposures. Several studies have suggested that inhaled or parenteral steroids are effective at decreasing respiratory complications after chlorine exposure.30,31 The proposed mechanism is decreased recruitment of inflammatory mediators and immune cells, as well as increased stimulation of surfactant producing pneumocytes.32 One animal model showed a benefit of inhaled budesonide if administered within 30 minutes of exposure. This same study showed a significant decrease in improvement if treatment was delayed to 60 minutes, which suggests that steroids should be administered soon after an exposure when feasible.33Nebulized sodium bicarbonate may be another adjunctive treatment for chlorine pulmonary exposures. Theoretically, inhaled bicarbonate can neutralize hypochlorous and hydrochloric acids, decreasing severity of lung injury. One non-randomized, placebo-controlled study of chlorine exposure reported improved pulmonary function tests (PFT), but patient-oriented benefits and long-term outcomes were not determined.34 Another study examining nebulized sodium bicarbonate revealed improved subjective symptoms, with no reported adverse effects.35 In both studies patients received concomitant inhaled beta-agonists and intravenous steroids. Another case series utilized bicarbonate without beta-agonists or steroids in patients with mild chlorine exposures. All three patients subjectively improved after treatment with a 3.75% sodium bicarbonate nebulizer and no adverse effects were reported.36 The utility of nebulized bicarbonate has not been firmly established, and the optimal dose has also not been delineated. A reasonable dose is 3.75–5% nebulized over 20 minutes and may be repeated. A 3.5% solution can be prepared by taking 2 mL of a 7.5% intravenous preparation of sodium bicarbonate solution (the concentration found in most cardiac “crash carts”) and combining with 2 mL of normal saline. Because precipitates can form if combined, it is important that nebulized sodium bicarbonate be administered separately from nebulized albuterol sulfate.37 Another theoretical treatment is the administration of an anti-oxidant such as N-acetyl cysteine (NAC). Although some data from animal models exists, there is currently not enough information to support the use of anti-oxidants in a clinical setting.32,38
Some patients will present with minimal or no symptoms. A small percentage who are minimally symptomatic on presentation may go on to develop delayed pulmonary edema over a period of several hours.4 Therefore, patients with mild symptoms should be observed for eight to 24 hours for delayed onset of respiratory complications.17 Patients who are asymptomatic after their exposure can be discharged after six hours of observation, but must be counseled on symptoms that should prompt a return for further care.
Disaster and Mass Casualty (MASCAL) Considerations
Personnel moving into areas of higher concentration or prolonged exposure time will need appropriate HAZMAT equipment for respiratory and skin protection. First responders must be equipped with and trained to use this PPE prior to a major chlorine release. Because of the potential for large numbers of exposed patients who may require treatment and monitoring, a hospital can become overwhelmed. It is important that hospitals and municipalities have mutual-aid agreements that coordinate the transfer of medications, equipment and personnel to the areas they are most needed. Especially important is respiratory support equipment. During the previously mentioned event in South Carolina, 10% of the hospitalized patients required intubation and mechanical ventilation.3 Additionally, incident command must help coordinate patient triage and transportation to appropriate medical facilities. Equitable distribution of patients based on each hospital’s surge capacity can minimize the strain on individual hospitals.
Triage is an ongoing process, and must be repeated as patients move through the levels of decontamination. The initial level of decontamination is the “hot zone,” which is typically the actual incident location. The “warm zone” is geographically distant from the incident and is frequently the entry point to decontamination. This area can restrict patient flow and may be an appropriate time to re-triage. As patients complete the decontamination process, they pass from the “warm zone” to the “cold zone.” Upon reaching this area, patients may need to be re-triaged again, then directed to the appropriate level of care.39
A critical challenge to MASCAL incident command is maintaining clear and regular communication. People working or living in areas being evacuated must be notified of the need to leave, as well as safe evacuation routes that avoid exposure. The general population must be notified of signs and symptoms of chlorine exposure and then directed to triage and care areas for the “walking wounded.” Hospitals nearest the incident scene must be prepared to receive most of the “walking wounded,” as well as the most critical patients. There must also be a point of contact for friends and families seeking missing loved ones. The best mechanism for the spread of such information will vary, depending on the size and demographics of a given community.
SUMMARY
In addition to the occult HAZMAT threat, the intentional use of chlorine as an unconventional weapon is now occurring. Large chlorine stores in the U.S. are vulnerable and lack adequate security. Operation Iraqi Freedom has demonstrated multiple events of successful use of weaponized chlorine transport vehicles, resulting in hundreds of casualties. Successful management of a chlorine MASCAL incident requires increased awareness and planning. As for all HAZMAT responses, proper equipment and training for first responders and a network of supporting medical facilities are needed to provide adequate care. Management of chlorine exposure involves decontamination and treatment of potential pulmonary injuries. Beta-agonists can be helpful for bronchospasm. Nebulized bicarbonate may decrease symptoms and prevent lung injury. Early corticosteroid use may play a role in treating lung inflammation and, possibly, preventing post-injury scarring. Severe cases may require endotracheal intubation and mechanical ventilation. While all these measures may prove useful in the treatment of patients after a chlorine exposure, further research is needed to delineate the optimal treatment regimen. Recognizing the evolving threat posed by chlorine, both in the form of an accidental release as well as an unconventional weapon, is an important first step to being prepared for this type of incident.
Footnotes
Supervising Section Editor: Colleen Buono, MD
Submission history: Submitted March 25, 2009; Revision Received July 7, 2009; Accepted October 19, 2009
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Address for Correspondence: Robert Jones, MD, Department of Emergency Medicine, Madigan Army Medical Center, ATTN: MCHJ-EM, Tacoma, WA 98431
Email: robert.jones66@us.army.mil
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Brennan RJ, Waeckerle JF, Sharp TW, et al. Chemical warfare agents: emergency medical and emergency public health issues. Ann Emerg Med. 1999;34:191–204. [PubMed]
2. Gussow L. Disaster Scenario: Tons of Chlorine Transported by U.S. Rail. Emergency Medicine News. 2007:18.
3. Van Sickle D, Wenck MA, Belflower A, et al. Acute Health effects after exposure to chlorine gas released after a train derailment. Am J Emerg Med. 2009;27:1–7. [PubMed]
4. Wenck MA, Van Sickle D, Drociuk D, et al. Rapid assessment of exposure to chlorine released from a train derailment and resulting health impact. Public Health Rep. 2007;122:784–92.[PMC free article] [PubMed]
5. Wald M, Hart A. “Ninth Victim of Chlorine Leak Is Found.” New York TimesJanuary 9, 2005. Available at: http://www.nytimes.com/2005/01/09/national/09train.html Accessed December 2, 2007.
6. Gordon S. Emergency Plan Missing Key DetailsAvailable at: http://www.thenewstribune.comAccessed December 2, 2007.
7. Gordon S. “Thousands Could Have Been Exposed to Deadly Gas on Tacoma’s Tideflats.” The News TribuneMarch 9, 2008. Available at:http://www.thenewstribune.com/2008/03/09/304409/thousands-could-have-been-exposed.htmlAccessed April 6, 2008.
8. Bach L, Walton B. “Las Vegas Dodged a Bullet: Chlorine-hauling Tanker Rolls Free.” Las Vegas Review-JournalApril 30, 2007. Available at: http://www.lvrj.com/news/9466232.html Accessed December 2, 2007.
9. Bach L. “Rail Tanker Escape Reviewed.” Las Vegas Review-JournalOctober 25, 2007. Available at:http://www.lvrj.com/ Accessed December 2, 2007.
10. Tawfeeq M. “Scores Choke in Poison Gas Attack.” Cable News NetworkFebruary 20, 2007. Available at: http://edition.cnn.com/2007/WORLD/meast/02/20/iraq.main Accessed March 19, 2008.
11. Parsons C. “Chlorine Truck Blast Kills Five in Iraq.” AlertNetFebruary 20, 2007. Available at:www.Reuters.com Accessed March 19, 2008.
12. Cave D, Fadam A. “Iraq Insurgents Employ Chlorine in Bomb Attacks.” New York TimesFebruary 22, 2007. Available at: http://www.nytimes.com/2007/02/22/world/middleeast/22iraq.htmlAccessed December 2, 2007.
13. Semple K. “Suicide Bombers Using Chlorine Gas Kill 2 and Sicken Hundreds in Western Iraq.” New York TimesMarch 18, 2007. Available at:http://www.nytimes.com/2007/03/18/world/middleeast/18iraq.html?fta=y Accessed December 2, 2007.
14. Rubin A. “Chlorine Gas Attack by Truck Bomber Kills Up to 30 in Iraq.” New York TimesApril 7, 2007. Available at: http://www.nytimes.com/2007/04/07/world/africa/07iht-web-0407-iraq.5182467.html Accessed December 2, 2007.
15. Parrish JS, Bradshaw DA. Toxic inhalational injury: gas, vapor and vesicant exposure. Respir Care Clin N Am. 2004;10:43–58. [PubMed]
16. Greenfield RA, Brown BR, Hutchins JB, et al. Microbiological, biological, and chemical weapons of warfare and terrorism. Am J Med Sci. 2002;323:326–40. [PubMed]
17. Traub S. Respiratory Agent Attack (Toxic Inhalation Injury) In: Ciottone G, editor. Disaster Medicine. 3rd ed. Philadelphia: Mosby; 2006. pp. 573–581.
18. Evans RB. Chlorine: state of the art. Lung. 2005;183:151–67. [PubMed]
19. Agency for Toxic Substances and Disease Registry Medical Management Guideline: Chlorine. Available at: http://www.atsdr.cdc.gov/MMG/MMG.asp?id=198&tid=36 Accessed July 8, 2008.
20. Department of Labor: Occupational Safety and Health Administration (OSHA) TABLE Z-1 Limits for Air Contaminants (1910.1000 TABLE Z-1). Available at: www.osha.gov Accessed January 21, 2009.
21. Winder C. The toxicology of chlorine. Environ Res. 2001;85:105–14. [PubMed]
22. Batchinsky AI, Martini DK, Jordan BS, et al. Acute respiratory distress syndrome secondary to inhalation of chlorine gas in sheep. J Trauma. 2006;60:944–56. [PubMed]
23. Ploysongsang Y, Beach BC, DiLisio RE. Pulmonary function changes after acute inhalation of chlorine gas. South Med J. 1982;75:23–6. [PubMed]
24. Department of Health and Human Services: Agency for Toxic Substances and Disease Registry (ATSDR) Medical Management Guideline: Chlorine. Available at: http://www.atsdr.cdc.gov Accessed July 8, 2008.
25. Horton D, Berkowitz Z, Kaye W.Department of Health and Human Services: Agency for Toxic Substances and Disease Registry (ATSDR). The Public Health Consequences From Acute Chlorine Releases, 1993–2000. Available at: http://www.atsdr.cdc.gov Accessed January 20, 2009.
26. (OSHA). Department of Labor: Occupational Safety and Health Administration. General description and discussion of the levels of protection and protective gear (1910.120, App B). Available at: www.osha.gov Accessed August 7, 2008.
27. U.S. Army Medical Research Institute of Chemical Defense CCCD In: eds. Medical Management of Chemical Casualties Handbook 3rd ed2000.
28. Noltkamper D, O’Malley G. CBRNE-Lung Damaging Agents, ChlorineAvailable at:www.emedicine.com Accessed September 10, 2008.
29. Villar J, Kacmarek RM, Perez-Mendez L, Aguirre-Jaime A. A high positive end-expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial. Crit Care Med. 2006;34:1311–8. [PubMed]
30. Gunnarsson M, Walther SM, Seidal T, et al. Effects of inhalation of corticosteroids immediately after experimental chlorine gas lung injury. J Trauma. 2000;48:101–7. [PubMed]
31. Wang J, Winskog C, Edston E, et al. Inhaled and intravenous corticosteroids both attenuate chlorine gas-induced lung injury in pigs. Acta Anaesthesiol Scand. 2005;49:183–90. [PubMed]
32. Baker DJ. Critical care requirements after mass toxic agent release. Crit Care Med. 2005;33:S66–74. [PubMed]
33. Wang J, Zhang L, Walther SM. Inhaled budesonide in experimental chlorine gas lung injury: influence of time interval between injury and treatment. Intensive Care Med. 2002;28:352–7.[PubMed]
34. Aslan S, Kandis H, Akgun M, et al. The effect of nebulized NaHCO3 treatment on “RADS” due to chlorine gas inhalation. Inhal Toxicol. 2006;18:895–900. [PubMed]
35. Bosse GM. Nebulized sodium bicarbonate in the treatment of chlorine gas inhalation. J Toxicol Clin Toxicol. 1994;32:233–41. [PubMed]
36. Vinsel PJ. Treatment of acute chlorine gas inhalation with nebulized sodium bicarbonate. J Emerg Med. 1990;8:327–9. [PubMed]
37. Howard C, Ducre B, Burda AM, et al. Management of chlorine gas exposure. J Emerg Nurs.2007;33:402–4. [PubMed]
38. Leustik M, Doran S, Bracher A, et al. Mitigation of chlorine-induced lung injury by low-molecular-weight antioxidants. Am J Physiol Lung Cell Mol Physiol. 2008;295:L733–43.[PMC free article] [PubMed]
39. Caneva D. Chapter 62: Chemical Attack. In: Ciottone G, editor. Disaster Medicine. 3 ed. Philadelphia: Mosby Elsevier; 2006.


