| Author | Affiliation |
|---|---|
| Sean O. Henderson, MD | Department of Emergency Medicine, Keck School of Medicine of the University of Southern California Department of Preventive Medicine, Keck School of Medicine of the University of Southern California |
| Vannita Simma-Chiang | Department of Emergency Medicine, Keck School of Medicine of the University of Southern California |
| Chi Lee, MD | Department of Emergency Medicine, Keck School of Medicine of the University of Southern California |
| Kirsten Calder, MD | Department of Emergency Medicine, Keck School of Medicine of the University of Southern California |
| Wendy J. Mack, PhD | Department of Preventive Medicine, Keck School of Medicine of the University of Southern California |
ABSTRACT
Introduction:
We examined the effect of two β2-adrenoreceptor (β2AR) polymorphisms (A46G and C79G) in asthmatics presenting to the Emergency Department (ED) in relation to their response to standard therapy measured by change in Forced Expiratory Volume at one second (FEV1). Our hypothesis was that the polymorphisms in the β2AR gene would predict clinical response to therapy with 46G and 79C displaying decreased response to inhaled therapy.
Methods:
This was a pilot feasibility study of a convenience sample of patients seen in the ED for acute exacerbation of asthma. Baseline data collected included: age, gender, ethnicity, vital signs, baseline FEV1, body mass index (BMI), smoking history and medications taken prior to arrival to the ED. Patients received standard ED care and FEV1 was measured after each treatment. Blood was taken and genotyped.
Results:
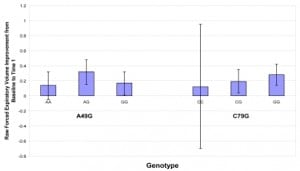
Fifty-three patients were enrolled over a three-month period. Using mean improvement in FEV1 from baseline to the first treatment as the primary outcome of interest, we performed multivariable linear regression analyses, with the FEV1 change as the dependent variable. When modeled as an ordinal covariate representing the number of G alleles present, there was a significant positive trend for the C79G locus (p=0.035). Those who were GG homozygotes had a 0.284 L/min improvement in FEV1 (31%) after their initial albuterol treatment compared to 0.123 L/min (12%) in those who were CC homozygotes. This represents a 2.5 times relative difference and a 19% actual difference. Genotypes at the A46G locus were not associated with FEV1 change.
Conclusion:
In this pilot study of ED patients with acute asthma exacerbation, there was a significant effect of genotype on response to therapy.
INTRODUCTION
Asthma accounts for more than 1.5 million Emergency Department (ED) visits, one-third of whom are admitted, and more than 5,500 (0.4%) deaths per year.1 In the setting of acute asthma exacerbation in the ED, inhaled β2-adrenergic agonists, such as albuterol, are the mainstay of treatment. Unfortunately, wide variation exists in how individual patients respond to therapy, a phenomenon well known to emergency physicians. The finding that there are common functional genetic variants of the β2- adrenoreceptor (β2AR) has led to the suggestion that response to therapy may vary from individual to individual depending upon their genotypic makeup.2,3,4,5,6
Single nucleotide polymorphisms (SNPs) are common, single-base pair variations in the DNA. There are some 1.4 million SNPs in the human genome, 60,000 of which are in coding regions. The β2AR gene is located on the long arm of chromosome 5, and thirteen SNPs have been identified in the gene. Two closely linked coding polymorphisms at amino acid positions 16 (A46G) and 27 (C79G) are common in the general population and in controlled outpatient trials have demonstrated to modify the phenotypic response to β2- agonists (46G and 79C being associated with decreased response to inhaled therapy).2
In this feasibility study, we examined these two SNPs in an asthmatic population presenting for acute care in the ED. Our goal was to determine whether different genotypes at these two genetic loci affected response to β2-agonists as measured by forced expiratory volume at one second (FEV1).
This was an IRB-approved feasibility study of a convenience sample of patients seen in the ED for acute exacerbation of previously diagnosed asthma. Patients for this study were recruited in the ED of a large urban facility serving a local population of some 1.5 million individuals. Patients meeting inclusion criteria were consented and baseline data were obtained, including, age, ethnicity, height (cms) and weight (kg), BMI (kg/m2), smoking history (current, past, never), medication used prior to arrival, and past medical history. An initial set of vital signs including blood pressure (mmHg), respiratory rate and pulse rate as well as pulse oximetry (%) was collected. Prior to the initiation of therapy we also measured FEV1 using incentive spirometery (MicroSpirometer) with the best of three consecutive measurements.
Patients then received standardized care with albuterol-inhaled therapy, receiving 5.0 mg via a hand-held nebulizer every 20 minutes for a total of four treatments. Patients also received 60 mg of prednisone P.O. after the first inhaled treatment. Patients were not administered any other inhaled medications until after completion of the study protocol. Immediately after each inhaled treatment, the patient was reexamined and FEV1 measurements were obtained.
Study patients also underwent phlebotomy to obtain one 10ml green top tube (heparinized vacutainer) for genotype testing. For processing, we utilized an automated specimen component dispensing machine (the Cryo-Bio System). DNA extraction was accomplished on an ongoing basis using the Qiagen 96 DNA Blood Biorobotic Kit.
Genotyping assays were performed using the Taqman assay. Allele-specific probes for use in the TaqMan assay were designed for each of the polymorphic sites within the genes of interest. The oligonucleotide primers for amplification of the polymorphic region are: 5′: CCCAGCCAGTGCGCTTACCT and 3′: CCGTCTGCAGACGCTCGAAC (18). β2AR A(46)G probes used to detect each of the alleles are: GCACCCAATGGAAGCCATG and GCACCCAATACAAGCCATG (21). β2AR C(79)G probes used to detect each of the alleles are: GTCACGCAGCAAAGGGACG and GTCACGCAGGAAAGGGACTG (21).
Statistical Analysis
Outcome variables included five separate measurements (one measurement prior to treatment and four measurements after treatment 20 minutes apart) of FEV1. Data analysis related the degree of improvement in FEV1 to the polymorphisms seen in the β2-adrenoreceptor at the two loci in question—A46G (AA homozygyotes, AG heterozygotes, and GG homozygotes) and C79G (CC, CG, and GG). Demographic variables including, age, gender, ethnicity, smoking history, height, weight, body mass index, and medication profile were also considered in our analysis.
Our analyses consider the absolute difference between baseline FEV1 (immediatelybefore the first inhaled treatment) and that measured at time #1 (immediately afterthe first inhaled treatment) as the primary outcome measure of interest since it was noted in exploratory analyses that subsequent FEV1s (at post-treatments #2 – 4) were highly correlated with the first post-treatment measurement, FEV1 #1 (p-value for all <0.0001 with Pearson correlation coefficients ranging from 0.92 – 0.96).
We used analysis of variance to test for differences in the mean FEV1 change among the genotypes at each locus. To adjust for potentially confounding covariates, we also performed multivariable linear regression analyses, with the FEV1 change (first post-treatment minus baseline) as the dependent variable. The independent variables included the two genetic loci as well as the covariates listed above. In the regression model, the genotypes were modeled as the number of ‘G’ alleles present (0,1,2).
RESULTS
Fifty-three patients were enrolled over a three-month period (Table 1). Twenty-one of these were male and the mean age was 39.7 years (range 19–57 years). Mean baseline FEV1 was 1.17 L/min (range 0.33 to 2.59 L/min) and improved to a mean of 1.42 L/min (range 0.29 – 3.15) after the first inhaled treatment. Our preliminary analyses revealed that particular polymorphisms were highly correlated such that the presence of a given allele at one locus could predict the allele at the other locus (i.e., the two were in linkage disequilibrium). Specifically, individuals homozygous for the ‘A’ allele at locus 46 were in all instances homozygous for the ‘G’ allele at locus 79.
Those individuals who were 79GG homozygotes had a 0.284 L/min improvement in FEV1 (31%) after their initial albuterol treatment compared to 0.123 L/min (12%) in those who were CC homozygotes. This represents a 2.5 times relative difference and a 19% actual difference (Figure 1). When modeled as an ordinal covariate representing the number of ‘G’ alleles present, there was a significant positive trend for FEV1 change with increasing numbers of ‘G’ alleles at the C79G locus (p=0.035). Genotypes at the A46G locus were not associated with FEV1 change in either regression model.
DISCUSSION
β-agonists are the most commonly prescribed asthma medications and the mainstay of therapy in the treatment of acute exacerbation in the ED.8 The candidate gene approach, which has proved so challenging for complex disease processes such as hypertension, diabetes and cancer, has been fairly successful in identifying variation involved in the treatment of asthma. SNPs have been identified that have pharmacologic implications with regards to response to β2 agonists, muscarinic antagonists, 5-LOX inhibitors, CysLT1 antagonists, glucocortocoids, and theophylline.6 Two of the multiple β2-agonists SNP’s (A46G and C79G) described in the literature are more common than the others and thus, we believe, more clinically relevant.2,6,7
The first polymorphism, A46G, has been linked to higher levels of receptor down regulation after exposure to long-term β2-agonist therapy.3,4 Studies have shown that patients with a ‘G’ allele have increased nocturnal asthma symptoms, higher airway reactivity, and decreased response to β2-agonist therapy.5 In the setting of an acute asthma exacerbation, these patients may need to resort to alternative forms of management such as corticosteroids or anticholinergics. It has also been suggested that individuals who die due to their asthma represent a group of 46G homozygotes, brittle asthmatics with desensitization of their β2 receptor.9
The second polymorphism, C79G, appears to serve in protection against desensitization and/or down regulation of the receptor.3,4 Patients with the 79G polymorphism exhibit decreased bronchial hyperactivity. Of note, when both the 46G and 79G polymorphisms were present, 46G activity was dominant over 79G in down regulating β2-adrenoreceptors and more prevalent in moderate vs. mild asthmatics.10
In our study, as patients increased the number of copies of 79C in their β2AR gene, their response to inhaled therapy of β2-agonists decreased by almost 20%. Interestingly, we were not able to demonstrate an effect of the A46G locus on patients’ response to albuterol therapy as measured by FEV1. One explanation for this may be tachyphylaxis in these acutely stressed asthmatics. Given that the β2AR in these patients is most likely “pre-desensitized” by endogenous epinephrine and norepinephrine, the lack of a measurable difference is not unexpected.
LIMITATIONS
The primary limitation of this pilot study is the small number of individuals who were 79C homozygotes. In addition the results may have been influenced by the large percentage of patients who received medications prior to contact with EMS or the ED. Their response to therapy may have been attenuated by these prior medications.
CONCLUSION
In this study of acute asthmatics we found a significantly higher rate of improvement for those patients who were 79GG homozygotes in the β2AR gene when compared to 79CC homozygotes. While the need to have information about a patient’s genotype in an acute care setting such as the ED may not be self-evident, it is the promise of effective patient-specific therapies and the hope of obtaining prognostic information from a patient’s genotype that appeals most to the physicians involved in the acute care of patients. Such information may allow a goal-directed approach for individual patients and will allow the practitioner to predict the clinical course over the initial two- to four-hour time period of emergency care. In the setting of asthma, the long term potential of such information may be measured by fewer intubations, fewer hospitalizations and fewer deaths.1
Footnotes
Submission history: Submitted May 6, 2007; Accepted June 9, 2007.
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Address for correspondence: Sean O. Henderson, MD, Department of Emergency Medicine, LAC + USC Medical Center, 1200 N. State Street Room 1011, Los Angeles, CA 90033
Email: sohender@hsc.usc.edu
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Crystal RG. Research opportunities and advances in lung disease. JAMA.2001;285:612–618. [PubMed]
2. Liggett SB. Polymorphisms of the β2-adrenergic receptor and asthma. Am J Respir Crit Care Med. 1997;156:S156–S162. [PubMed]
3. Green SA, Turki J, Hall IP, Liggertt SB. Amino-terminal polymorphisms of the human β2– adrenergic receptor impart distinct agonist-promoted regulatory properties.Biochemistry. 1994;33:414–419.
4. Green SA, Turki J, Bejarano P, Hall IP, Liggett SB. Influence of β2-adrenergic receptor genotypes on signal transduction in human airway smooth muscle cells. Am J Respir Cell Mol Biol. 1995;13:25–33. [PubMed]
5. Aziz I, Hall IP, McFarlane LC, Lipworth BJ. β2-adrenoreceptor regulation and bronchodilator sensitivity after regular treatment with formoterol in subjects with stable asthma. J Allergy Clin Immunol. 1998;101:337–341. [PubMed]
6. Fenech A, Hall IP. Pharmocogenetics of asthma. Br J Clin Pharmacol. 2002;53:3–15.[PMC free article] [PubMed]
7. Ligget SB. β2 – Adrenergic receptor pharmacogenetics. Am J Respir Crit Care Med.2000;161:S197–S201. [PubMed]
8. Beveridge RC, Grunfeld AF, Hodder RV, Verbeek PR. Guidelines for the emergency management of asthma in adults. CMAJ. 1996;155:25–37. [PMC free article] [PubMed]
9. Tan S, Hall IP, Dewar J, Dow E, Lipworth B. Association between β2-adrenoreceptor polymorphism and susceptibility to bronchodilator desensitization in moderately severe stable asthmatics. Lancet. 1997;350:995–999. [PubMed]
10. Weir TD, Mallek N, Sandford AJ, Bai TR, Awadh N, Fitzgerald JM, Cockcroft D, James A, Liggett SB, Pare PD. β2-adrenergic receptor haplotypes in mild, moderate and fatal/near fatal asthma. Am J Respir Crit Care Med. 1998;158:787–791. [PubMed]