| Author | Affiliation |
|---|---|
| Phillip J. Goebel, MD | San Antonio Uniformed Services Health Education Consortium, Department of Emergency Medicine, San Antonio, TX Mike O’Callaghan Federal Hospital, Department of Emergency Medicine, Nellis AFB, NV |
| Justin B. Williams, MD | San Antonio Uniformed Services Health Education Consortium, Department of Emergency Medicine, San Antonio, TX |
| Robert T. Gerhardt, MD, MPH | San Antonio Uniformed Services Health Education Consortium, Department of Emergency Medicine, San Antonio, TX U.S. Army Institute of Surgical Research, Fort Sam Houston, TX Uniformed Services University of the Health Sciences, Department of Military and Emergency Medicine, Bethesda, MD |
ABSTRACT
Introduction:
To determine if a sensitive D-dimer assay can exclude progression to organ dysfunction, death, and intensive care unit (ICU) admission in patients presenting to the emergency department (ED) with suspected infection, and if increasing levels of D-dimer are predictive of those end points.
Methods:
The study took place at two academic EDs, both located in tertiary care hospitals. This was a prospective convenience sample of adult patients presenting with an infective process and at least two of four criteria for the Systemic Inflammatory Response Syndrome. We measured D-dimer levels in the participants and abstracted their records for the end points. Sensitivity and specificity were calculated and receiver operating characteristic analysis was performed to determine if a higher cutoff would have a greater specificity for our end points.
Results:
We enrolled 134 patients. Twelve were excluded from analysis (10 for lack of a D-dimer, one for recent surgery, and one for complete loss to follow up). Using the cutoff of 0.4 established by our laboratories as positive, the D-dimer had a sensitivity of 94% (CI95; 76–99) for organ dysfunction in the ED, 93% (72–99) for organ dysfunction at 48 hours, 93% (81–98) for ICU admission, and 100% (63–100) for 30-day mortality. However, at this cutoff, specificity was not statistically significant. Significantly raising the cutoff for a positive resulted in a decrease in sensitivity but improved specificity.
Conclusion:
This study was limited by its nonconsecutive patient recruitment and sample size. A normal D-dimer may exclude progression to organ dysfunction, ICU admission, and death and, at higher cutoff levels, could help risk stratify patients presenting to the ED with signs of sepsis.
INTRODUCTION
Background
Patients presenting to the emergency department (ED) in septic shock have been shown to benefit from early goal-directed therapy aimed at improving perfusion1 and early antibiotic therapy.2 Our tools are currently limited in predicting which ED patients with an infection, but without overt sepsis or organ dysfunction, will progress to severe sepsis, shock, or death. The Systemic Inflammatory Response Syndrome (SIRS) criteria, while part of the definition of sepsis, are not adequately sensitive or specific to be used alone to predict the clinical course of a patient.3 A predictive biomarker could be helpful to clinicians to risk-stratify infected patients to an appropriate level of care.
Importance
As a candidate biomarker of sepsis, fibrin D-dimer stands apart in its availability in the ED. It has demonstrated sensitivity for sepsis in ICU patients.4,5 Additionally, it has been correlated with other potential markers of sepsis and is a potential predictor of mortality and organ system failure.6–9 Shilon et al.10 demonstrated an association between levels of D-dimer and severity of disease in hospitalized patients with community-acquired pneumonia. These studies have focused primarily on patients requiring ICU-level care and have not examined a broader patient population, therefore limiting application of the data to ED patients. However, not all studies have shown the D-dimer to be useful early in the course of sepsis. Iba et al.11 in 2007 failed to show a difference in D-dimer levels on Day 0, but a difference appeared on Day 2. In an ED pilot study, D-dimer had a sensitivity of only ∼61–67% for patients who ultimately were found to have positive blood cultures.12 This study used a semi-quantitative D-dimer assay and only looked at blood culture results without looking at clinical outcomes. If the early correlation of D-dimer levels with illness severity described in ICU patients could be reproduced in the ED population, the D-dimer could be used to better risk stratify patients with infections into appropriate levels of care.
Goals of This Investigation
We sought to determine if the D-dimer is an appropriate test in the initial evaluation for sepsis. Our primary hypotheses are that the D-dimer is adequately sensitive to exclude organ dysfunction, ICU requirement, and mortality in patients presenting with clinical presentations consistent with sepsis, and that higher levels of D-dimer are predictive of organ dysfunction, ICU requirement, and death. We considered a sensitivity ≥ 90% clinically important and desired to demonstrate 95% confidence intervals of 10% or less. A test with these characteristics would potentially help determine which patients (who typically would be admitted to a ward level of care) would benefit from more intensive monitoring or aggressive therapy.
METHODS
Study Design
We performed a prospective, observational study using a combination of prospective laboratory analysis and chart auditing to investigate the correlation between a positive D-dimer and the end points of hospital admission, initial ICU admission, organ dysfunction, and 30-day mortality. Our sample size was calculated to be 116 patients, based on the estimate of a relative risk of 5.5 for mortality in patients with an elevated D-dimer compared to those with normal D-dimers,7 and 50% of the patients having positive D-dimers.
Setting
All patients in the study visited the EDs at two tertiary-care military medical centers from August 2007 through March 2008. The institutional review board approved and monitored the study at both institutions.
Selection of Participants
All adult patients presenting to the ED regardless of mode of arrival with a suspected infection (radiographic, laboratory, or clinical findings indicating a need for antibiotics), as determined by the treating team’s attending physician and/or third year resident and at least two out of four SIRS criteria (Table 1), were eligible for enrollment. The presence of two or more of the four SIRS criteria was established as a criterion to focus the study on a higher acuity level of infection consistent with the diagnostic criteria for sepsis (Table 2). Patients with a history of thromboembolic disease, recent surgery, or those in basic military training (due to issues with follow up and ethical issues centered on ability to consent) were excluded. Pregnant women and patients with cancer were not excluded from the study. Patients were identified, consented, and enrolled as a convenience sample by senior resident and attending physicians in the ED at the time of presentation. Next of kin or powers of attorney were allowed to provide consent in incapacitated patients.


Data Collection
Patients who agreed to participate in the study had blood samples sent separately from their clinical lab work for measurement of their D-dimer levels. All laboratory work including the D-dimer assay was performed by the institutions’ regular lab facilities. The treating teams did not have access to the D-dimer results unless they had ordered a separate D-dimer study as part of their clinical evaluation. To evaluate end organ dysfunction we ordered a complete metabolic panel, complete blood count, PTT, PT, and serum lactate as part of the study. The results from these studies were available to the ED and admitting teams. Data from the ED encounter (SIRS criteria, suspected infection, and disposition) were entered on a standard data sheet by the enrolling physician. ICU admission was determined by the admitting service in conjunction with the emergency physicians. Generally, patients requiring vasopressor support, ventilator support, or close monitoring as determined by the admitting service were admitted to the ICU teams. The primary investigator then used the same sheet to abstract the patients’ ED and inpatient charts through the next 30 days for evidence of the primary end points. Laboratory results, including lactate, coagulation panels, chemistry panels, and blood counts, were abstracted as well. Patients were called at the end of the 30-day follow-up period to confirm that no other hospitalizations occurred outside the primary medical system. After all other variables were abstracted the D-dimer result was added to the datasheet. The primary investigator was not blinded to the study objectives during the abstraction phase.
Methods of Measurements
We used the Liatest D-dimer assay (Diagnostica Stago, Parsippany, New Jersey), a quantitative, microlatex agglutination test with a reference cut-off of 0.4 mg/dL as positive. The assay is run on venous blood collected in calcium citrate tubes. This assay is the same assay used in the evaluation of thromboembolic disease at both of our institutions. We utilized the Sepsis-related Organ Failure Assessment (SOFA) to determine the presence of organ dysfunction. The SOFA score uses measurements of cardiovascular, neurologic, coagulation, hepatic, renal, and respiratory function to create a composite score. We maintained the convention of a SOFA score of three or greater as positive for significant organ dysfunction.13 When explicit data needed to calculate a complete SOFA score were missing from the record the patient was assigned the least dysfunctional value for that system and were included in the data analysis. Mortality was determined using a combination of the inpatient and ED records and telephone follow up. After the 30-day follow up was completed, the primary investigator transferred the de-identified data into a password protected Microsoft Access (Microsoft, 2007) database.
Primary Data Analysis
We used SPSS 16.0 (SPSS, 2007) to compute descriptive statistics of the participants and to determine the sensitivity, specificity, odds ratio, positive likelihood ratio and negative likelihoods associated with a cut point of 0.4 mg/dL. We then constructed receiver operating characteristic (ROC) curves for each of the study end points, and a D-dimer cut point at ∼95% specificity was evaluated for its sensitivity, odds ratio, and positive and negative likelihood ratios. Selected randomly, 10% of the records (14) were abstracted by a second reviewer blinded to D-dimer results, and inter-rater variability was evaluated using the kappa statistic.
In a 1996 article Gilbert and Lowenstein described eight criteria of a quality retrospective review study. Those criteria are trained abstractors, explicit case-selection criteria, definition of variables, abstraction forms, meetings among abstractors and study coordinators, monitoring of abstractor performance, blinding of abstractors to study goals, and testing of interrater agreement.1,4 We used a single abstractor, who was not blinded to the study objectives, but remained blinded to D-dimer results until after the other data points were abstracted. Selection criteria and variable definition were explicitly defined a priori. Abstractor performance was monitored through the blinded re-abstraction of a random 10% of the charts by a blinded second abstractor and then calculating interrater reliability with a kappa statistic. Other than the lack of complete blinding of the primary abstractor, Gilbert and Lowenstein’s criteria were met.
RESULTS
We enrolled 134 patients in the study from August 2007 to March 2008. Twelve patients were excluded from data analysis, 10 for lack of a D-dimer (due to hemolysis or loss of the sample), one for a recent surgery, and one for complete loss to follow up. Demographic data is presented in Table 3. There were no discrepancies between the abstractors in the recording of the primary variables of interest, (admission, ICU admission, organ dysfunction in the ED, organ dysfunction at 48 hours, organ dysfunction during the 30-day follow up, in-hospital death, and 30-day mortality), giving a kappa of 1.0 (95% CI; 0.5–1.0).
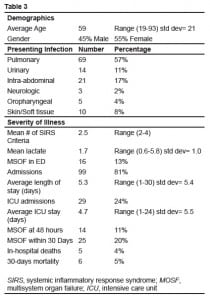
Figures 1 and and22 show the outcomes of patients entered in to the study. Thirty-day mortality was 5%, 24% were admitted to the ICU, and 20% had SOFA scores greater than three at any point in the 30-day follow-up period.
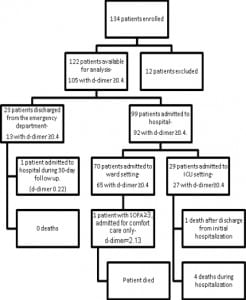
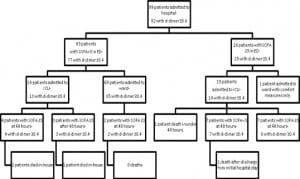
Retrospectively using quality improvement data collected separately from our study, we estimate that 60 patients were admitted to the ICU with sepsis through both of our EDs during the study period and we were only able to enroll 29. If the same proportion of ambulatory and ward patients was missed, then we were only able to enroll an estimated 50% of the eligible patients.
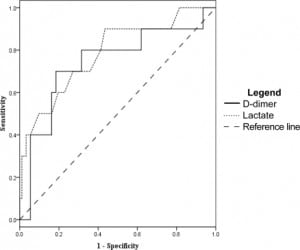
The D-dimer was associated most closely with the need for ICU level care. At a level of 0.4 mg/dL the sensitivity was 93% (95% CI 81–98) and specificity was 16% (95% CI 12–18). However, other measures of association did not reach statistical significance. Odds ratio was 2.6 (95% CI 0.62–11), positive likelihood ratio 1.1 (95% CI 0.93–1.2), and negative likelihood ratio was 0.43 (95% CI 0.11–1.5). After ROC analysis (Figure 3) we found that 95% specificity was achieved with a D-dimer level of 4.0 mg/dL. At this cut off sensitivity was 35% (95% CI 23–43), the odds ratio was 9.3 (95% CI 2.9–29), the positive likelihood ratio was 6.4 (95% CI 2.5–17), and the negative likelihood ratio was 0.69 (95%CI 0.58–0.85).
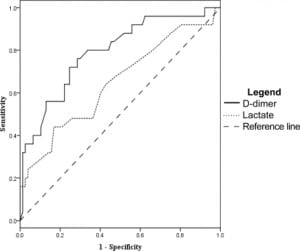
Using the D-dimer to evaluate which patients would have a SOFA score greater than three at 48 hours from admission gave a sensitivity of 93% (95% CI 72–99) and a specificity of 15% (95% CI 12–16). Other measures of association did not reach statistical significance, with an odds ratio of 2.3 (95% CI 0.35–14), positive likelihood ratio of 1.1 (95% CI 0.82–1.2), and a negative likelihood ratio of 0.48 (95% CI 0.08–2.3) at D-dimer cut off of 0.4 mg/dL. ROC analysis (Figure 4) gave a D-dimer level of 7.0 mg/dL as 94% specific, with a sensitivity of 29% (95% CI 13–47), odds ratio 6.8 (95% CI 1.7–27), positive likelihood ratio of 5.1 (95% CI 1.7–15), and negative likelihood ratio of 0.76 (95% CI 0.55–0.95)
For 30-day mortality, a D-dimer level of 0.4 mg/dL or greater gave a sensitivity of 100% (95% CI 63–100), failing to achieve our desired degree of precision. The remaining tests were not significant with a specificity of 15% (95% CI 13–15), an odds ratio of ∞ (95% CI 0.25-∞), a negative likelihood ratio of 0 (95% CI 0–2.9), and a positive likelihood ratio of 1.2 (CI 95% 0.73–1.2). Using the ROC curve (Figure 5), a 95% specificity for 30-day mortality occurred at a D-dimer level of 9.0 mg/dL, and had a sensitivity of 17% (CI 95% 3–52), an odds ratio of 3.1 (95% CI 0.44–24), a positive likelihood ratio of 2.8 (95% CI 0.46–12), and a negative likelihood ratio of 0.89 (CI 95% 0.51–1).
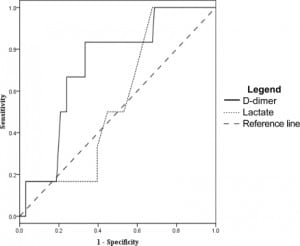
Lactate levels were available in 102 of the 122 patients. We generated ROC curves using the data from these 102 patients for the end points of ICU admission, organ dysfunction at 48 hours, and 30- day mortality. These curves are shown in Figures 3–5 for comparison with the curves for the D-dimer data.
DISCUSSION
Our results suggest that the D-dimer could be used as a screening test in the ED to exclude significant organ dysfunction, intensive care requirement, and possibly mortality in patients with suspected infection and SIRS. While the values for the sensitivity of the test were above our 90% threshold, the confidence intervals remained too wide to definitively state that the test is adequate to use by itself in determining the disposition of patients from the ED. At the typical cut off for normal (0.4 mg/dL) the odds ratio, positive likelihood ratio, negative likelihood ratio and specificity failed to reach significance.
In this study the D-dimer performed better as a positive predictor at cut points 10–20 times greater than normally used for thromboembolic disease. Specifically in the setting of organ dysfunction, the test might provide useful clinical positive predictive power. These odds ratios at the higher cutoffs were in a range that could allow the test to be used alone or in a panel of tests to increase or decrease the pretest probability. However, the value of the useful cut point was different for different end points, and requires prospective validation in a larger study to be of use clinically.
Notably, the D-dimer was elevated in the nine patients who had not yet developed organ dysfunction, but who went on to do so later in their hospital course (Figure 2). Patients who demonstrate severe sepsis or shock early in their hospital course generally have more severe organ dysfunction, but they may have a decreased mortality rate and decreased length of stay in the ICU when compared to patients who develop sepsis later in their hospital course.15 Utilizing the D-dimer in the early testing of patients with suspected sepsis may help to identify these patients earlier in their illness.
LIMITATIONS
The primary limitation of our study is its sample size and convenience patient sampling. We estimate that only 50% of patients eligible to be enrolled during the study period were enrolled, and this reflects in the wide confidence intervals for the test characteristics computed. Particularly, this lack of power limits our ability to comment on the ability of the D-dimer to exclude or predict mortality.
Additionally, not all data points were available for all patients. This may have lead to an underestimation of the patients’ SOFA scores and a resulting underestimation of the number of patients suffering from organ dysfunction. This underestimation of the degree of organ dysfunction likely would skew our results away from confirming our hypotheses given the number of patients with elevated D-dimers.
We kept our study population relatively broad with no exclusion criteria for patients with renal disease, cancer, pregnancy, or advanced age to better mirror an actual ED population. These populations can have higher D-dimer levels than a young healthy population and represent a possible confounding variable in our results.
Our lack of universal screening for thromboembolic disease creates a potential confounder. One patient in this study was found to have a pulmonary embolism during a subsequent hospitalization. She was diagnosed with pneumonia and sepsis syndrome during her initial evaluation and her blinded D-dimer was 1.85 at that time. One week later, she returned to the ED and was diagnosed with a pulmonary embolism by CT angiography. Given the large percentage of D-dimers in our study, positive D-dimers are not likely to be useful in distinguishing pneumonia from pulmonary embolism. Ordering angiograms solely on the basis of a D-dimer in our population would lead to overutilization of ionizing radiation.
CONCLUSION
Our preliminary study demonstrated that the fibrin D-dimer is a potentially sensitive diagnostic test for use in the exclusion of organ dysfunction, need for intensive care unit admission and 30-day mortality with ROC curves similar to serum lactate, but has poor specificity at usual D-dimer thromboembolism cutoffs. As a confirmatory test for the presence of organ dysfunction the assay performed well enough at higher cut points (10–20 times normal) to potentially be used as a component of a model or scoring system, but not well enough to use as a stand-alone test. These higher cutoffs must be tested a priori in a larger patient sample to validate their potential use.
Footnotes
The authors would like to acknowledge the support of the Department of Clinical Investigations at Brooke Army Medical Center, and the Laboratory Support Squadron at Wilford Hall Medical Center, which provided the funding which made this study possible. The opinions or assertions expressed herein are the private views of the authors and are not to be construed as official or as reflecting the views of the U.S. Department of the Army, U.S. Department of the Air Force, or the U.S. Department of Defense.
Supervising Section Editor: Robert W Derlet, MD
Submission history: Submitted January 6, 2009; Revision Received October 14, 2009; Accepted October 25, 2009
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Address for Correspondence: Phillip Goebel, MD, Department of Emergency Medicine, 99 MDOS/SGOE, Mike O’Callaghan Federal Hospital, 4700 N Las Vegas BLVD, Nellis AFB, NV 89191
Email : phillip.goebel.md@gmail.com
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Rivers E, Nguyen B, Havstad S, et al. Early Goal Directed Therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77. [PubMed]
2. Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med.2006;34:1589–96. [PubMed]
3. Alberti C, Brun-Buisson C, Goodman SV, et al. Influence of Systemic Inflammatory Response Syndrome and Sepsis on Outcome of Critically Ill and Infected Patients. Am J Respir Crit Care Med.2003;168:77–84. [PubMed]
4. Kinasewitz GT, Yan SB, Basson B, et al. Universal changes in biomarkers of coagulation and inflammation occur in patients with severe sepsis regardless of causative micro-organism. Crit Care.2004;9(2):R92–100.
5. Deitcher SR, Eisenberg PR. Elevated concentrations of cross-linked fibrin degradation products in plasma. An early marker of gram-negative bacteremia. Chest. 1993;103:1107–12. [PubMed]
6. Kollef MH, Eisenberg PR, Shannon W. A rapid assay for the detection of circulation D-dimer is associated with clinical outcomes among critically ill patients. Crit Care Med. 1998;26:1054–60.[PubMed]
7. Shorr AF, Thomas SJ, Alkins SA, et al. D-dimer correlates with proinflammatory cytokines levels and outcomes in critically ill patients. Chest. 2002;121:1262–68. [PubMed]
8. Dhainaut F, Shorr AF, Macias WL, et al. Dynamic evolution of coagulopathy in the first day of severe sepsis: Relationship with mortality and organ failure. Crit Care Med. 2005;33:341–8.[PubMed]
9. Iba T, Kidokoro A, Fukunaga M, et al. Association between the severity of sepsis and the changes in hemostatic molecular markers and vascular endothelial damage markers. Shock. 2005;23(1):25–9. [PubMed]
10. Shilon Y, Shitrit AB, Rudensky B, et al. A rapid quantitative D-dimer assay at admission correlates with the severity of community acquired pneumonia. Blood Coagu Fibrinolysis.2003;14(8):745–8.
11. Iba T, Gando S, Murata A, et al. Predicting the severity of systemic inflammatory response syndrome (SIRS)-associated coagulopathy with hemostatic molecular markers and vascular endothelial injury markers. J Trauma. 2007;63(5):1093–8. [PubMed]
12. Quick G, Eisenberg P. Bedside measurement of D-dimer in the identification of bacteremia in the emergency department. J Emerg Med. 2000;19(3):217–23. [PubMed]
13. Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European society of intensive care medicine. Intensive Care Med. 1996;22:708–10.
14. Gilbert EH, Lowenstein SR, KozioI-McLain J, et al. Chart reviews in emergency medicine research: Where are the methods? Ann Ernerg Med. 1996;27:305–308.
15. Roman-Marchant O, Orellana-Jimenez CE, De Baker D, et al. Septic shock of early or late onset. Does it matter? Chest. 2004;126:173–8. [PubMed]


