| Author | Affiliation |
|---|---|
| John C. Stein, MD | University of California, San Francisco |
| Sergio Hernandez, MD | University of California, San Francisco |
| Anke Hebig, BA | University of California, San Francisco |
ABSTRACT
Introduction:
Acute dermatologic conditions are a concern for acute care practitioners. Comprising 1.4% of presenting complaints to emergency departments, most skin complaints are relatively benign; however, some conditions can be quite severe. Prompt diagnosis is essential to avoid unnecessary morbidity and mortality. To review drug-induced vasculitis.
Case:
We present the case of a 43-year-old female with a chief complaint of bruising to her ear, arm, and leg. She was found to have necrotizing vasculitis induced by propylthiouracil.
Conclusion:
In this case, we look at the highlights of this presentation and review key aspects of cutaneous vasculitis for the practicing emergency provider.
INTRODUCTION
Acute dermatologic conditions are a concern for all healthcare professionals. With the increased national utilization of acute care services, emergency physicians must have a basic knowledge of such disorders. In 2004, “skin rash” complaints accounted for 1.4% of all emergency department (ED) visits.1 The majority of these conditions are benign and non-emergent, with diagnoses ranging from contact dermatitis to psoriasis. However, a small number of patients present with acute dermatologic conditions requiring rapid diagnosis and treatment.
Due to the multiple etiologies associated with dermatologic emergencies, diagnosis may often be difficult. However, one of the most common causes of dermatologic presentations to acute care facilities is a drug reaction, and it has been estimated that 10–20% of cutaneous reactions to drugs are vasculitic reactions.2,3 The prognosis for drug-induced vasculitis is usually improved with prompt removal of the offending medication.4 We present a case in which a 43-year-old woman with a history of Graves’ disease presented to the ED after two weeks of progressive palpable purpura caused by a propylthiouracil-induced vasculitis.
CASE REPORT
A 43-year-old woman presented to the ED with a chief complaint of new-onset bruising. She had been well until two weeks prior to admission, when she developed symptoms consistent with an upper respiratory tract infection (URI). At that time the patient developed a bruise on her left calf without any history of trauma. As this started to resolve she noted some bruising on the right ear four days later. She complained that she was developing bruises along her right tricep associated with increasing redness, swelling and pain over these lesions. The patient denied any history of domestic violence, liver disease, prior bruising episodes, family history of bleeding disorders, heavy menses, or anticoagulant use. Systemically the patient denied any fevers, chills, chest pain, shortness of breath, abdominal pain, hematuria or joint complaints. Her past medical history was significant for Graves’ disease, which was being followed by her endocrinologist. She also reported degenerative joint disease of her left knee. Her medications included 50-mg propylthiouracil (PTU) each day, which she has been taking for the past nine years. She also reported the use of 400mg Motrin as needed for pain relief from her arthritis. The patient denied taking any other medications including over-the-counter medications for her recent URI symptoms. The patient stated that her mother had recently been diagnosed with diabetes and thyroid disease but denied any other family history. The patient reported a 20 pack/year tobacco history but denied any illicit drug use.
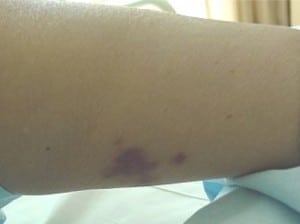
On arrival to the ED she was afebrile at 36.4°C, with a blood pressure of 116/78 mmHg, heart rate at 74 beats per minute, respirations at 14 breaths/minute with oxygen saturation at 97% on room air. On examination, she was a well-appearing woman in no acute distress. Her skin examination showed several findings. She had trace ecchymosis along the superior aspect of the helix of the right ear (Figure 1). This area was otherwise non-tender to palpation with no other abnormalities noted at the ear. She had a second ecchymotic area at her right tricep approximately 4 × 10 cm in diameter (Figure 2). The arm was tender andindurated, with some erythema at the borders of the lesion. There were no areas of fluctuance, and the neurovascular function of the arm and hand were intact. She also had one small left calf ecchymosis which was superficial and non-tender, approximately 1.5cm in diameter (Figure 3). Regarding the remainder of the examination, the patient’s eyes were normal, pupils were equally round and reactive to light, and no conjunctival injection was noted. The fundoscopic examination was unremarkable bilaterally. Lungs were clear to auscultation without increase in respiratory effort. The cardiac exam showed regular rate and rhythm without murmur, rub, or gallop. The abdominal examination was non-tender with no evidence of rebound or guarding. The rectal examination was normal with guiac-negative brown stool. The neurologic examination was unremarkable.

Skin lesion at right pinna.
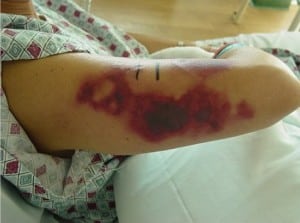
Skin lesion at right tricep.
Skin lesion at left lower calf.
Laboratory analyses of CBC, coagulation studies, electrolytes, urinalysis, liver function tests, and negative urine pregnancy test were unremarkable. Blood cultures were sent and eventually returned negative. Erythrocyte sedimentation rate was elevated at 54. The patient was admitted to the hospital for further, assessment and subsequent lab studies were as follows with normal ranges in parentheses. Thyroid function tests showed a TSH of 1.40 uIU/mL, a free T4 of 0.74 ng/dL (0.85–1.69), and a free T3 of 4.23 pg/mL (2.0–4.1). Antiphospholipid panel was negative. Anti-nuclear antibody screen was negative. Perinuclear-staining anti-neutrophil cytoplasmic antibodies (P-ANCA) was positive. Antithrombin III was 104% (80–120), D-dimer was 1303ng/mL (<250), and fibrinogen was 345 mg/dL (240–490).
The clinical presentation of a palpable, purpuric rash in conjunction with elevated erythrocyte sedimentation rate and ANCA titers indicated a vasculitis. In general, the differential diagnosis for vasculitis includes infectious etiologies (22.1%), autoimmune disorders (4.4%), connective tissue disease (11.4%), malignancy (4.3%), environmental exposures including medications (22.7%) or idiopathic (39% frequency).5 In our patient, the lack of associated clinical symptoms and absence of other possible etiologic sources lead us to believe the ANCA-positive vasculitis was secondary to her PTU. Previous data suggests that vasculitic complications associated with PTU administration have a varied time of onset and have been reported anywhere from three days to seven years from the time of initiation of therapy.6 Furthermore, despite conservative management with 60 mg prednisone per day, the patient’s symptoms did not resolve until several days after admission when the PTU therapy was discontinued. The decision to continue her PTU while awaiting the diagnostic workup, rather than immediately discontinue the medication, was made due to her lack of other end-organ failure often associated with vasculitis. With the vasculitis resolving, the patient was treated with a total course of oral prednisone, 60 mg each day for 10 days, along with metoprolol, 100mg each day for her Graves’ disease, and she was discharged from the hospital. The possibility of re-challenging the patient on PTU was subsequently considered after her symptoms had resolved, but given the data (albeit limited) which suggests a high recurrence of vasculitis, it was felt that other treatment options should be pursued.6
The patient returned for subtotal thyroidectomy to address her Graves’ disease. The surgery was uneventful, and she was discharged with supplementation for hypothyroidism, hypocalcemia, and hypomagnesemia.
DISCUSSION
Vasculitis is a rare condition among the general population with one study showing an estimated lifetime incidence of biopsy-proven vasculitis at 39.6 per million; however, the number varies based on geography, tertiary vs. outpatient setting.7 The true incidence may be higher due to the under-diagnosis of mild or subclinical presentations. The etiology of vasculitic disease can be attributed to a primary idiopathic process or to a secondary autoimmune syndrome, infectious agent, trauma, or medication. In attempting to determine an etiology, a proper history is essential. In general, drug-induced vasculitis is more likely to have cutaneous manifestation compared to idiopathic vasculitis.8 And it is more likely to manifest with pulmonary and renal complications than musculoskeletal complaints.9
Drug-induced vasculitis has been reported from penicillins, sulfonamides, allopurinol, antithyroid drugs, and multiple other compounds.10 Of the thyroid drugs propylthiouracil is the most often reported as causing drug-induced vasculitis. Ithas also been associated with other rheumatic manifestations, including serum sickness and PTU-induced lupus.9 There is some debate whether PTU is the primary cause of rheumatologic disease or if the increased incidence is due to the underlying thyroid disease of the patients, which has been shown to predispose patients to other autoimmune conditions. PTU has been associated with various other inflammatory conditions, such as agranulocytosis, aplastic anemia, hepatitis, interstitial pneumonitis, and others.
The mechanism of PTU-induced vasculitis is not clear. Studies have shown that PTU can accumulate in neutrophils. This may allow PTU to bind to myeloperoxidase (MPO), which when oxidized can inactivate this enzyme. This inactivation with the continued presence of PTU can induce the formation of auto-antibodies, thereby stimulating other neutrophils to degranulate, causing vascular damage.4,6,10,11,12 Clinically the development of the MPO-ANCA antibodies are more prevalent in the serum of patients treated with PTU, and studies have demonstrated that MPO-ANCA positivity was increased after being treated by PTU as compared to other hyperthyroid medications.9,11,13 It should be noted, however, that not all patients with these antibodies developed clinical symptoms of PTU-induced rheumatic disease, which contraindicates the use of these auto-antibodies as a screening tool.14
There are currently 44 cases of PTU-induced vasculitis in the literature since 2002, with an apparent female and Japanese-ethnic predominance.6 This gender predominance is likely attributed to the higher incidence of thyroid disease in women. It is unknown why Japanese ethnicity, which accounts for half of all reported cases, predisposes patients.11There may be a genetic predisposition to the formation of auto-antibodies, or it may be caused by variations in treatment regimens. The age of onset varies but appears to have a higher incidence in younger patients; however, the reported age range is 13–80 years old.6,14 Of note, the development of PTU-induced vasculitis is hypothesized to be independent of the dosage of PTU prescribed or the duration of PTU treatment, based on the limited data available.6
The clinical presentation can be varied and complex due to systemic complications and multi-organ involvement of small-vessel disease. Signs of PTU-induced vasculitis include anemia (seen in the majority of patients), skin lesions (ulcers, purpura, subcutaneous nodules, and erythema nodosum-like and erythema multiforme-like lesions), arthralgia, fever, malaise, lymphadenopathy, hematuria/proteinuria, alveolar hemorrhage, and pleural effusion.4,6,14 Multiple organ systems can be affected, including renal failure, cholestatic jaundice, cardiac dysfunction, pleural effusions, pneumonitis/pleuritis, and hearing loss. The most common laboratory abnormalities include neutropenia, anemia, thrombocytopenia, and elevated transaminases10 (although our patient demonstrated none of these). Histologically the majority of patients developed crescentic glomerulonephritis or other forms of renal changes.4,14
Clinical diagnosis is often difficult because of the overlapping presentations of the various vasculitic diseases. Although biopsy is diagnostic, in most cases the diagnosis of drug-induced vasculitis is made by exclusion or with resolution of symptoms after withdrawal of medications. Once PTU-induced vasculitis is determined, the simple withdrawal of PTU usually causes resolution of the symptoms after 1–4 weeks.3 Some patients have been reported to have continued laboratory abnormalities (elevated serum creatinine, proteinuria, positive ANCA titers) throughout their clinical follow-up without evidence of clinical symptoms.3 Patients with more severe presentations are typically treated with steroids. When considering treatment for Gravesí disease after resolution of their symptoms, re-administration of PTU is not recommended because it often causes a repeat reaction;6,10,11 however, there does not appear to be cross-reactivity between agents.2,10Therefore, consideration of treatment should be a multi-disciplinary approach to weigh the risk and benefits of further medical treatment versus surgical intervention.
CONCLUSION
Drug-induced vasculitis should be considered in any patient who takes medications and develops cutaneous eruptions. Emergency practitioners should be able to differentiate benign skin conditions from those that are potentially progressive, causing morbidity and possibly mortality.
Footnotes
Supervising Section Editor: Christopher A. Kahn, MD, MPH
Submission history: Submitted January 23, 2008; Revision Received June 1, 2008; Accepted August 5, 2008
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Address for Correspondence: John C. Stein MD, Department of Emergency medicine, University of California, San Francisco, 505 Parnassus, Box 208, San Francisco, CA 94143
Email: jstein@emergency.ucsf.edu
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. McCaig L, Nawar E. National hospital ambulatory medical care survey: 2004 emergency department survey. CDC Advance Data. Vital and Health Statistics.2006;372
2. Dubost JJ, Souteyrand P, Sauvezie B. Drug-induced vasculitides. Baillieres Clin Rheumatol. 1991;5:119–138. [PubMed]
3. Ekenstam E, Callen JP. Cutaneous leukocytoclastic vasculitis. Clinical and laboratory features of 82 patients seen in private practice. Arch Dermatol. 1984;120:484–489.[PubMed]
4. Otsuka S, Kinebuchi A, Tabata H, Yamakage A, Yamazaki S. Myeloperoxidase-antineutrophil cytoplasmic antibody-associated vasculitis following propylthiouracil therapy. Br J Dermatol. 2000;142:828–830. [PubMed]
5. Carlson JA, Ng BT, Chen KR. Cutaneous vasculitis update: diagnostic criteria, classification, epidemiology, etiology, pathogenisis, evaluation and prognosis. Am J Dermatopathol. 2005;27:504–528. [PubMed]
6. ten Holder SM, Joy MS, Falk RJ. Cutaneous and systemic manifestations of drug-induced vasculitis. The Annals of Pharmacotherapy. 2002;36:130–147. [PubMed]
7. Watts RA, Jolliffe VA, Grattan CE, Elliot J, Lockwood M, Scott DG. Cutaneous vasculitis in a defined population—clinical and epidemiological associations. J Rheumatol.1998;25:920–924. [PubMed]
8. Wiik A. Clinical and laboratory characteristics of drug-induced vasculitic syndromes.Arthritis Research & Therapy. 2005;7:191–192. [PMC free article] [PubMed]
9. Aloush V, Litinsky I, Caspi D, Elkayam O. Propylthiouracil-induced autoimmune syndromes: two distinct clinical presentations with different course and management.Semin Arthritis Rheum. 2006;36:4–9. [PubMed]
10. Hardy OT, Smolinski KN, Yan AC, Grimberg A. PTU-associated vasculitis in a girl with turner syndrome and graves’ disease. Pediatric Emergency Care. 2006;22:52–54.[PubMed]
11. Sato H, Hattori M, Fujieda M, Shigetaka S, Inomata H, Hoshi M, et al. High prevalence of antineutrophil cytoplasmic antibody positivity in childhood onset graves’ disease treated with propylthiouracil. The Journal of Clinical Endocrinology & Metabolism.2000;85:4270–4273. [PubMed]
12. Sheen YS, Chu CY, Yu HS. Antineutrophil cytoplasmic antibody–positive cutaneous leukocytoclastic vasculitis associated with propylthiouracil therapy. Arch Dermatol.2006;142:879–880. [PubMed]
13. Harper L, Chin L, Daykin J, Allahabadia A, Heward J, Gough SC, et al. Propylthiouracil and carbimazole associatedantineutrophil cytoplasmic antibodies (ANCA) in patients with Graves’ disease. Clinical Endocrinology. 2004;60:671–675.[PubMed]
14. Nakamori Y, Tominaga T, Inoue Y, Shinohara K. Propylthiouracil (PTU)-induced vasculitis associated with antineutrophil antibody against myeloperoxidase (MPO-ANCA)Internal Medicine. 2003;42:529–533. [PubMed]


