| Author | Affiliation |
|---|---|
| Brian Rice, MDCM | University of Southern California, Department of Emergency Medicine, Los Angeles, California |
| Phillips Perera, MD | University of Southern California, Department of Emergency Medicine, Los Angeles, California |
ABSTRACT
Cysticercosis is an emerging disease in the United States. Neurocysticercosis may rarely cause disease within the spinal cord, but the occurrence of such pathology can produce debilitating symptoms for patients. We present the second report in the literature of intramedullary spinal neurocysticercosis presenting with a Brown-Sequard syndrome.
CASE REPORT
A 42-year-old Mexican-born male with no past medical or surgical history presented to a Los Angeles emergency department with complaints of 1 month of progressive left lower extremity numbness and right lower extremity stiffness limiting his ability to walk. He reported no history of trauma, as well as no systemic complaints of fevers, chills, weight loss, or night sweats.
A detailed neurologic examination of the cranial nerves and upper extremities was completely normal. The examination of the right lower extremity showed hyperreflexia and 4/5 strength for all muscle groups. The left lower extremity had diminished sensation to noxious stimulation from the inguinal crease to the plantar surface of the foot. A noncontrast computed tomography of the head was initially performed and yielded no significant findings. An emergent thoracolumbar spine magnetic resonance imaging (MRI) was then ordered for further evaluation.
The MRI revealed a heterogenous intermedullary mass in the spinal cord at the T10 to T11 level with mild enhancement and mass effect (Figure). The mass was localized to the left lateral aspect of the cord, with the cord displaced to the right. Significant edema was noted on the right lateral aspect of the cord. The initial differential included cavernoma and ependymoma. The patient was admitted to the hospital and urgent neurosurgery was performed.
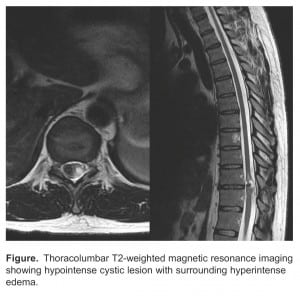
Thoracolumbar T2-weighted magnetic resonance imaging showing hypointense cystic lesion with surrounding hyperintense edema.
The patient underwent a laminectomy at T10 to T11, and 2 distinct cystic structures—one left lateral and superficial and one more deeply invested—were identified and removed from the cord. Intraoperatively, the patient was tested for somatosensory evoked potentials and motor-evoked potentials on his right lower extremity. They were initially diminished but improvement was noted as early as the time of closure. Pathology reports identified the cysts as neurocysticercosis. The patient recovered well from surgery and was ambulating with improved strength at the time of discharge to a rehabilitation facility.
DISCUSSION
Cysticercosis is caused by the parasite Taenia solium and is the most common parasitic disease worldwide.1 Its larval stage begins in pigs, passes to humans via undercooked pork, and then develops into an adult within humans. There the adult tapeworm eventually produces eggs, which pass throughout the body, lodge into tissues, and cause symptomatic cysts.2 Neurocysticercosis is the central nervous system (CNS) manifestation of this disease and is the most common parasitic infection of the CNS worldwide.3 Cysticercosis is endemic in Mexico and Latin America (as well as areas of Africa, India, and Asia) but is an emerging disease in the United States with up to 1,000 new cases a year being diagnosed.4 These cases are predemoniantly in the Southwest owing largely to immigration from endemic areas.5 In Los Angeles in particular, the disease burden is so heavy that up to 10% of adults receiving neuroimaging for a new onset seizure in the emergency department were diagnosed with neurocysticercosis.6
Although neurocysticercosis is common, only 1% to 5% of cases demonstrate spinal cord involvement.7 Of those cases, leptomeningeal involvement is 6 to 8 times more common than the intramedullary disease our patient displayed.8 The results of a recent literature review indicate that this patient represents the 55th reported case of intramedullary neurocysticercosis in the literature.9 Manifestation of this lesion as Brown-Sequard syndrome is even rarer, with a review of the literature yielding only a single other report.10
Brown-Sequard syndrome was described in 1850 by the neurologist Charles-Édouard Brown-Séquard to describe the clinical syndrome accompanying hemisection of the spinal cord.11 The classic syndrome involves “crossed” findings, with hemiplegia, hyperreflexia, and loss of light touch and proprioception affecting the ipsilateral side, and sensory defects of painful touch and temperature affecting the contralateral side. This asymmetrical presentation results from the crossing of neural fiber tracts at different levels within the CNS.
The 3 distinct neural tracts that travel within the cord are the corticospinal tract, the dorsal columns, and the spinothalamic tract. The corticospinal tract carries upper motor neurons, which originate in the brain and decussate high up in the cervicomedullary junction to travel distally down the spine to provide motor control. The dorsal, or posterior, columns transmit light touch and proprioceptive information and also cross proximally in the CNS, at the level of the medulla. The spinothalamic tract conveys pain and temperature information. This tract has a different anatomic pathway from the corticospinal and posterior column tracts, with decussation distally in the CNS, rather than high within cervicomedullary region. This is usually via the anterior white commissure, 1 to 2 spinal segments above the dermatome it enervates.12
When the cord is hemisected, all 3 pathways are disrupted to give the classic syndrome. In clinical practice, complete transection is rare and partial transection with incomplete Brown-Sequard syndrome is more common.13 The transection of the corticospinal tract causes hemiplegia ipsilateral to the lesion, accompanied by hyperreflexia due to the loss of regulatory upper motor neuron control. The posterior columns also carry information to the ipsilateral side and account for the loss of prorioception and light touch to the same side as the cord lesion when severed. The spinothalamic tracts remain uncrossed until the distal spinal cord. When a lesion causes hemisection, pain and temperature sensation are lost on the contralateral side, at a level 1 to 2 segments below the crossed motor symptoms. Bladder function, which receives bilateral autonomic innervation, is typically spared by the hemisection.12
Our patient had all the symptoms of Brown-Sequard syndrome: ipsilateral motor weakness, hyperreflexia, and loss of proprioception and light touch, with a contralateral sensory deficit starting at L1, 2 segments below the T10 to T11 lesion. Interestingly, the right-sided tracts were disrupted in this case despite the cyst being on the left. The right side was affected by edema and mechanical pressure, known to cause neural disruption.14It was these factors that caused the tracts to be disrupted, rather than the slow-growing cyst.
LIMITATIONS
This is an isolated case of a rare disease. The cystic lesion occurred on the left lateral cord and the clinical syndrome implicated only the right-sided tracts. Edema and displacement were seen on the right and are known to causes neuronal disruption, yet the possibility remains that the cyst on the left contributed to the overall clinical picture.
CONCLUSION
Brown-Sequard syndrome is a classic but rare entity. It remains a topic in medical education owing to the complex neuroanatomy displayed through its clinical manifestations. It is overwhelmingly associated with penetrating trauma, but nontraumatic causes, such as neoplasm and infection, are reported and do need to be considered. Neurocysticercosis is an evolving medical reality in North America that is largely tied to immigration from endemic areas. This case not only represents an exceedingly rare infectious etiology of this classic syndrome, but also reminds us that physicians must remain aware of the emerging patterns of disease in the patients they treat and evolve their differential diagnoses to reflect these patterns.
Footnotes
Supervising Section Editor: Sean Henderson, MD
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Address for Correspondence: Brian Rice, MDCM, University of Southern California, Department of Emergency Medicine, 2051 Marengo St, Inpatient Tower – Rm C1A100, Los Angeles, CA 90033
E-mail: rice.brian@gmail.com
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Hawk MW, Shahlaie K, Kim KD, et al. Neurocysticercosis: a review. Surg Neurol.2005;63:123–132. [PubMed]
2. Garcia HH, Del Brutto OH. Taenia solium cysticercosis. Infect Dis Clin North Am.2000;14:97–119. [PubMed]
3. Psarros TG, Zouros A, Coimbra C. Neurocysticercosis: a neurosurgical perspective.South Med J. 2003;96:1019–1022. [PubMed]
4. Singhi P, Singhi S. Neurocysticercosis in children. J Child Neurol. 2004;19:482–492.[PubMed]
5. Kraft R. Cysticercosis: an emerging parasitic disease. Am Fam Physician. 2007;76:91–96. [PubMed]
6. Ong S, Talan DA, Moran GJ, et al. Neurocysticercosis in radiographically imaged seizure patients in U.S. emergency departments. Emerg Infect Dis. 2002;8:608–613.[PMC free article] [PubMed]
7. Garg RK, Nag D. Intramedullary spinal cysticercosis: response to albendazole: case reports and review of literature. Spinal Cord. 1998;36:67–70. [PubMed]
8. Queiroz LDS, Filho AP, Callegaro D, et al. Intra medullary cysticercosis: case report, literature review and comments on pathogenesis, J Neurol Sci. 1975;26:61–70. [PubMed]
9. Qi B, Ge P, Yang H, et al. Spinal intramedullary cysticercosis: a case report and literature review. Int J Med Sci. 2011;8:420–423. [PMC free article] [PubMed]
10. Torabi AM, Quiceno M, Mendelsohn DB, et al. Multilevel intramedullary spinal neurocysticercosis with eosinophilic meningitis. Arch Neurol. 2004;61:770–772.[PubMed]
11. Brown-Séquard C.-É. De la transmission croisée des impressions sensitives par la moelle épinière. Comptes rendus de la Société de biologie. 1851;2:33–44. (1850)
12. Eisen A. Anatomy and localization of spinal cord disorders. 2011.http://www.uptodate.com UpToDate Web site. Available at: . Accessed September 7,
13. Kobayashi S, Yoshizawa H, Yamada S. Pathology of lumbar nerve root compression, part 1: intraradicular inflammatory changes induced by mechanical compression. J Orthop Res. 2004;22:170–179. [PubMed]
14. Schreiber D. Spinal cord injuries. 2011. http://emedicine.medscape.com Medscape Web site. Available at: Accessed September 16.


