| Author | Affiliation |
|---|---|
| Erica Frumin, MD | University of California at Irvine School of Medicine, Irvine, California |
| Joelle Schlang, BS | University of California at Irvine School of Medicine, Irvine, California |
| Warren Wiechmann, MD, MBA | University of California at Irvine School of Medicine, Irvine, California |
| Stacy Hata, BS | University of California at Irvine School of Medicine, Irvine, California |
| Sasha Rosen, BS | University of California at Irvine School of Medicine, Irvine, California |
| Craig Anderson, PhD, MPH | University of California at Irvine School of Medicine, Irvine, California |
| Laura Pare, MD | University of California at Irvine School of Medicine, Irvine, California |
| Mark Rosen, MD | University of California at Irvine School of Medicine, Irvine, California |
| John Christian Fox, MD | University of California at Irvine School of Medicine, Irvine, California |
Introduction
Methods
Results
Discussion
Limitations
Conclusion
ABSTRACT
Introduction
The accurate diagnosis of elevated intracranial pressure (eICP) in the emergent setting is a critical determination that presents significant challenges. Several studies show correlation of sonographic optic nerve sheath diameter (ONSD) to eICP, while others show high inter-observer variability or marginal performance with less experienced sonographers. The objective of our study is to assess the ability of bedside ultrasound measurement of ONSD to identify the presence of eICP when performed by a single experienced sonographer. We hypothesize that ONSD measurement is sensitive and specific for detecting eICP and can be correlated with values obtained by external ventricular device (EVD).
Methods
This was a prospective blinded observational study conducted in a neurocritical care unit of a level 1 trauma center. ONSD measurement was performed on a convenience sample of 27 adult patients who required placement of an invasive intracranial monitor as part of their clinical care. One certified sonographer/physician performed all ultrasounds within 24 hours of placement of EVD. The sonographer was blinded to the ICP recorded by invasive monitor at the time of the scan. A mean ONSD value of ≥5.2 mm was taken as positive.
Results
The sonographer performed 27 ocular ultrasounds on individual patients. Six (22%) of these patients had eICP (EVD measurement of >20 mmHg). Spearman rank correlation coefficient of ONSD and ICP was 0.408 (p=0.03), demonstrating a moderate positive correlation. A ROC curve was created to determine the optimal cut off value to distinguish an eICP greater than 20 mmHg. The area under the receiver operator characteristic curve was 0.8712 (95% confidence interval [CI]=0.67 to 0.96). ONSD ≥5.2 mm was a good predictor of eICP (>20 mmHg) with a sensitivity of 83.3% (95% CI=35.9% to 99.6%) and specificity of 100% (95% CI=84.6% to 100%).
Conclusion
While the study suggests ONSD measurements performed by a single skilled operator may be both sensitive and specific for detecting eICP, confirmation in a much larger sample is needed. Ocular ultrasound may provide additional non-invasive means of assessing eICP.
INTRODUCTION
Elevated intracranial pressure (eICP) is common and potentially fatal. Timely diagnosis of this condition has a positive impact on morbidity and mortality, but the accurate evaluation of eICP presents a challenging diagnostic dilemma.1 The gold standard for diagnosis of eICP, external ventricular device (EVD), is highly invasive, unavailable in the initial assessment, and may be contraindicated in coagulopathic patients. Neuroimaging modalities, including computed tomography (CT) or magnetic resonance imaging (MRI), provide delayed diagnosis and are logistically difficult in agitated or unstable patients.2 Moreover, clinical diagnosis of eICP by emergency physicians has shown poor performance when compared to CT or optic nerve sheath diameter (ONSD) measurement.3
Physicians have observed and described the response of ONSD to changes in ICP extensively.4–6 The optic nerve is surrounded by subarachnoid cerebrospinal fluid, which is encased in the optic nerve sheath, an extension of the dura mater. As ICP increases, the subarachnoid space fills, resulting in an increased ONSD. The expansion is greatest at the retrobulbar terminating segment of the nerve.7 The expansion of the sheath is immediate and predictable.4,5,8 These characteristics suggest that ONSD is a sensitive and early marker for eICP and may provide particular utility for monitoring the changing status of critical patients.
The clinical application of this anatomical phenomenon shows promise for diagnosing eICP. Sonographic measurement of ONSD has been shown to be a useful predictor of eICP when compared to CT findings of eICP.3,9 Several studies have shown that ONSD is a good predictor of eICP as measured by EVD, while others have shown poor inter-rater reliability or inadequate image acquisition by less experienced operators.9–13
The range of proposed cut-off values for detection of eICP varies from 4.8 to 5.86 mm.11,13 The small dimensions of the ONSD, variation in sonographic technique, operator experience, and observation of artifacts all may contribute to the lack of consensus on an optimal cut-off value.
In this study a single, experienced sonographer performed all scans to eliminate the variable of multiple operators and determine whether a single operator using a standard technique can demonstrate whether ONSD can predict eICP. To the best of our knowledge, no studies thus far have examined the correlation between ONSD performed by a single experienced sonographer with direct ICP measurements from EVD.
METHODS
This was a blinded prospective observational study performed on a convenience sample of patients presenting with a variety of intracranial processes in the neurocritical care unit of an urban level 1 trauma center between September 16, 2008, and July 11, 2009. Subjects included adult patients with invasive intracranial monitors placed during weeks that the study physician was available to perform the scans. Consent was obtained from the patients or the appropriate surrogates and the local institutional review board at our hospital approved this study. We excluded patients with ocular trauma or a known history of ocular pathology.
Ocular ultrasounds were performed on a Sonosite M-turbo (SonoSite Inc., Bothell, WA) machine with a 5–10 MHz linear probe.15 One physician with RDMS certification and 3 years of clinical experience with sonography, including over 250 ocular ultrasound exams, performed all scans on patients in the supine position. Scans were performed on a closed eyelid with a generous amount of conductive gel. A linear probe was used to obtain an axial cross-sectional image of the optic nerve entering the fundus. The ultrasonographer fanned the globe superiorly and inferiorly to help visualize margins of the nerve. An average of 3 to 4 ultrasounds was required to obtain an optimal image with clear margins of the optic sheath. ONSD was measured from outer wall to outer wall 3 mm posterior to the globe.
Artifacts were avoided by instructing alert patients to look directly ahead to ensure that the visual axis was centered with the optic nerve running perpendicular to the fundus. The lids of unconscious patients were opened to assess globe axis. Only images taken with the optic nerve centered and with clear optic sheath margins were accepted. Clear images of the ONSD could be obtained for all patients. The sonographer accepted one measurement on each eye. The mean binocular ONSD was the outcome selected for statistical analysis. All measurements were obtained within 24 hours of EVD placement. Research associates recorded a single ICP measurement at the time of the scan and the study physician/ultrasonographer was blinded to the reading.
Research associats kept data logs and input the data to Stata (version 12.0, Stata Corp, College Station, TX). We performed a Shapiro-Wilk test to evaluate the ONSD and ICP distributions for normality, and produced a scatter plot to examine the data. A non-parametric Spearman rank correlation coefficient with a two-tailed p-value was used to assess for an association between the ONSD and ICP measurements. We assessed optimal cut-off value to detect ICP >20 mmHg, the pressure at which aggressive treatment usually begins, with a receiver operator characteristic (ROC) curve. We computed 95% confidence intervals (CIs) for sensitivity and specificity of potential cut-off values using exact statistics.
RESULTS
The sonographer performed 27 ocular ultrasounds on individual patients, 18 males and 9 females, with an average age of 45.6 years (range 18–76). Ten patients had hemorrhagic strokes (37%), 9 had subarachnoid hemorrhage (33%), 6 suffered blunt head trauma with various intracranial bleeds (22%), one had obstructive hydrocephalus (4%) and one had neurocysticercosis (4%). EVD showed eICP >20 cm H20 in 6 (22%) of the study participants. Clear images of the optic nerve could be obtained for all patients.
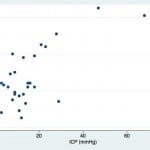
Although the expansion of ONSD is predictable, the relationship is not purely linear and may be asymptotic.4,5 The scatter plot produced by our data set supports this hypothesis (Figure 1). The Shapiro-Wilk test for normality demonstrated that neither ONSD (p=0.045) nor ICP (p=0.00002) had normal distributions. Given this finding, we assessed the relationship between ONSD and ICP using a non-parametric Spearman rank correlation coefficient, which produced a rho of 0.408 (p=0.03).
Figure 1. Scattergram of optic nerve sheath diameter (ONSD) versus intracranial pressure (ICP).
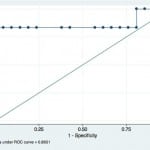
Because ONSD measurements were compared against invasive monitoring, a receiver operating characteristic (ROC) curve for eICP (>20 mmHg) was drawn to establish the optimal cut-off value to maximize sensitivity and specificity (Figure 2). The ROC curve demonstrated an area under the curve of 0.865 (95% CI=0.66 to 0.96). The ONSD cut-off value of ≥5.2 mm yielded the best test characteristics and accurately predicted eICP with a sensitivity of 83.3% (95% CI=35.9% to 99.6%) and specificity of 100% (95% CI=83.9% to 100%). The positive predictive value of ONSD ≥5.2 mm for eICP was 100% (95% CI=48% to 100%) and the negative predictive value of ONSD less than 5.2 mm was 95.5% (95% CI=77.2% to 99.9%).
Figure 2. Receiver operator curve for optic nerve sheath diameter as a test for intracranial pressure >20 cm water.
DISCUSSION
This study assessed the ability of sonographic measurement of ONSD by a single operator to identify the presence of eICP in patients with a wide variety of traumatic, medical, and infectious pathologies. Prior studies have only examined patients with traumatic injuries or hemorrhagic strokes.
We demonstrated that sonographic measurement of ONSD is sensitive (83.3%) and specific (100%) for detecting eICP and that this measurement can be correlated with values obtained by EVD when performed by a single sonographer. The high positive predictive value (100%) of this test suggests that it can be used as an additional tool to evaluate for eICP. Given the inherent risk of false-positive results when non-expert sonographers measure ONSD, we see the utility of ONSD measurements in decision-making, such as expediting CTs and calling for emergency neurology consultations prior to CTs.
We determined an optimal cut-off value of 5.2 mm by using EVD as the basis for comparison. This value was in line with prior studies.14 However, in the emergency department, where sensitivity is often more important than specificity, a lower ONSD threshold value may be more appropriate and would be a reasonable investigation for a follow-up study.
While the growing body of evidence suggests sonographic ONSD measurement has good predictive value for detecting eICP as measured by EVD, a few studies raise questions about the reproducibility of this exam.10–14 Quality of image acquisition and sonographic interpretation are both factors that vary with the level of expertise. As of yet, the level of experience needed for competency in performing the ONSD scan is undefined. It is possible that some of the variability in optimal cut-off values are secondary to measurement of artifact or the result of variability in the way the scan and measurements are performed suggesting that this technique should be more adequately described.11,13
Sonographic measurement of ONSD would not replace placement of EVD; however, it has the potential to diagnose eICP early when other means are unavailable or contraindicated. The tool is simple, available, cost-effective, and incurs no-harm to the patient, providing an additional non-invasive means of assessing eICP.
LIMITATIONS
Our study is limited by design in that one sonographer performed all observations with RDMS credentialing and extensive experience, limiting the generalizability of this study. However, this was a pre-hoc design element that was necessary to determine if the test was accurate in expert conditions prior to expanding to non-experts. Additionally, the threshold ONSD value of 5.2 mm reflects the characteristics of this cluster of 27 patients, as derived from an ROC curve. Although this may also limit the generalizability, this value was in line with previous studies.14
The study is also limited by its small size and wide 95% confidence interval (35.9% to 99.6%). The results should be validated in larger trials and at multiple centers. However, despite the small size, we believe that this sample represents an adequate spectrum of intracranial processes, including traumatic, hemorrhagic, obstructive, and infectious causes of eICP.
Additionally, we did not compare the performance of ONSD findings with the performance of common neuroimaging modalities (CT or MRI) for diagnosis of eICP, which, along with clinical picture, often serve as the basis for the decision to place invasive monitor. Future studies should consider making this comparison.
CONCLUSION
A non-invasive, inexpensive, rapid bedside assessment to detect eICP could aid in the early diagnosis of eICP, allowing for more rapid intervention. Measurement of ONSD has shown good performance for predicting eICP in this study as well as previous studies. As bedside ultrasound becomes widely available in emergency departments and critical care units, there is value in refining this technique and determining a universal cut-off to aid in this challenging diagnosis.
Footnotes
Address for Correspondence: Erica Frumin, MD. University of California, Irvine, Department of Emergency Medicine, 101 The City Drive Rt. 128-01, Orange, CA 92868. Email: efrumin@uci.edu. 3 / 2014; 15:217 – 220
Submission history: Revision received February 22, 2013; Submitted June 13, 2013; Accepted September 16, 2013
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Becker D, Miller J, Ward J. The outcome from severe head injury with early diagnosis and intensive management. J Neurosurg. 1977; 47:491-502.
2. Miller MT, Pasquale M, Kurek S. Initial head computed tomographic scan characteristics have a linear relationship with initial intracranial pressure after trauma. J Trauma. 2004; 56:967-972.
3. Tayal VS, Neulander M, Norton HJ, et al. Emergency department sonographic measurement of optic nerve sheath diameter to detect findings of increased intracranial pressure in adult head injury patients. Ann Emerg Med. 2007;49:508-514.
4. Hansen HC, Helmke K. Validation of the optic nerve sheath response to changing cerebrospinal fluid pressure: ultrasound findings during intrathecal infusion tests. J Neurosurg. 1997; 87:34-40
5. Hansen HC, Lagre W, Krueger O, et al. Dependence of the optic nerve sheath diameter on acutely applied subarachnoidal pressure –an experimental ultrasound study. Acta Ophthalmologica. 2011;89:e528-532.
6. Galetta S, Byrne SF, Smith JL Echographic correlation of optic nerve sheath size and cerebrospinal fluid pressure. J Clin Neuroopthalmol. 1989;9:79-82.
7. Hansen HC, Helmke K. The subarachnoid space surrounding the optic nerves: An ultrasound study of the optic nerve sheath. Surg Radiol Anat. 1996; 18:323-328.
8. Liu D, Kahn M. Measurement and relationship of subarachnoid pressure of the optic nerve to intracranial pressures in fresh cadavers. Am J Ophthalmol. 1993; 116:548-556.
9. Soldatos T, Karakitsos D, Chatzimichail K, et al. Optic nerve sonography in the diagnostic evaluation of adult brain injury. Critical Care. 2008;12:R67.
10. Kimberly HH, Shah S, Marill K, et al. Correlation of optic nerve sheath diameter with direct measurement of intracranial pressure. Acad Emerg Med. 2008; 15:201-204.
11. Geeraerts T, Merceron S, Benhamou D, et al. Non-invasive assessment of intracranial pressure using ocular sonography in neurocritical care patients. Intensive Care Med. 2008; 34:2062-2067.
12. Strumwasser A, Kwan RO, Yeung L, et al. Sonographic optic nerve sheath diameter as an estimate of intracranial pressure in adult trauma. J Surg Res. 2011; 170:265-271.
13. Rajajee V, Vanaman M, Fletcher JJ, et al. Optic nerve ultrasound for the detection of raised intracranial pressure. Neurocrit Care. 2011; 37:1059-1068.
14. Moretti R, Pizzi B, Cassini F, et al. Reliability of optic nerve ultrasound for the evaluation of patients with spontaneous intracranial hemorrhage. Neurocritical Care. 2009; 11:406-410.
15. Blaivas M, Theodoro D, Sierzenski PR. Elevated intracranial pressure detected by bedside emergency ultrasonography of the optic nerve sheath. Acad Emerg Med. 2003; 10:376-381.