| Author | Affiliation |
|---|---|
| Erik B. Kulstad, MD, MS | Advocate Christ Medical Center, Department of Emergency Medicine, Oak Lawn, IL |
| Ejaaz A. Kalimullah, MD | Advocate Christ Medical Center, Department of Emergency Medicine, Oak Lawn, IL |
| Karis L. Tekwani, MD | Advocate Christ Medical Center, Department of Emergency Medicine, Oak Lawn, IL |
| D Mark Courtney, MD | Feinberg School of Medicine, Northwestern University, Department of Emergency Medicine, Chicago, IL |
ABSTRACT
Despite its widespread use in North America and many other parts of the world, the safety of etomidate as an induction agent for rapid sequence intubation in septic patients is still debated. In this article, we evaluate the current literature on etomidate, review its clinical history, and discuss the controversy regarding its use, especially in sepsis. We address eight questions: (i) When did concern over the safety of etomidate first arise? (ii) What is the mechanism by which etomidate is thought to affect the adrenal axis? (iii) How has adrenal insufficiency in relation to etomidate use been defined or identified in the literature? (iv) What is the evidence that single dose etomidate is associated with subsequent adrenal-cortisol dysfunction? (v) What is the clinical significance of adrenal insufficiency or dysfunction associated with single dose etomidate, and where are the data that support or refute the contention that single-dose etomidate is associated with increased mortality or important post emergency department (ED) clinical outcomes? (vi) How should etomidate’s effects in septic patients best be measured? (vii) What are alternative induction agents and what are the advantages and disadvantages of these agents relative to etomidate? (viii) What future work is needed to further clarify the characteristics of etomidate as it is currently used in patients with sepsis? We conclude that the observational nature of almost all available data suggesting adverse outcomes from etomidate does not support abandoning its use for rapid sequence induction. However, because we see a need to balance theoretical harms and benefits in the presence of data supporting the non-inferiority of alternative agents without similar theoretical risks associated with them, we suggest that the burden of proof to support continued widespread use may rest with the proponents of etomidate. We further suggest that practitioners become familiar with the use of more than one agent while awaiting further definitive data.
When did concern over the safety of etomidate first arise?
Etomidate was first introduced into clinical practice in Europe in 1972 and was approved for use in the United States in 1983. In early June 1983, and later that same month, letters to the editor were published in The Lancet describing increased mortality in the setting of continuous sedation for trauma patients after the introduction of etomidate.1,2
In these reports Ledingham and Watt, in Glasgow, noticed that prior to etomidate introduction patient mortality ranged from 22–29%, whereas after etomidate introduction, mortality rose to 44%, despite no significant changes in the injury severity score (ISS) throughout the study period. Comparing patient outcomes from 1979 through 1980 (a total of 55 patients; mean age 55 years; ISS 24) with outcomes during 1981 through 1982 (a total of 88 patients; mean age 35 years; ISS 26), they found that despite the lower mean age of the latter group and a similar ISS, mortality increased from 25% when sedation agents were primarily morphine and benzodiazepines to 44% when sedation agents were primarily morphine and etomidate. Three quarters of the patients in each group were mechanically ventilated, and it was in these patients that the increased mortality appeared to occur. When patients in each group were stratified strictly by sedation agent, the results were even more striking. For all patients, mortality was 25% in patients not receiving etomidate and 69% in patients who received etomidate. When stratified by severity of illness using the ISS, the differences in mortality were as follows: ISS 10–20: 6% versus 50%; ISS 21–30: 36% versus 77%; and ISS greater than 30: 35% versus 100%. The authors noted that suppression of adrenocortical function, which had recently been reported in rats3 and critically ill patients,4, 5 might explain the apparent effect of etomidate. A footnote by the editor accompanying this report notes: “the company (Janssen) has agreed to cease promotion of the drug for sedation in intensive care.”
In a follow-up letter in late June 1983, the same group reported that their initial findings led to the end of etomidate use at their institution.1 Following this decision, they noted that 21 patients were treated with an alternative sedative agent, 15 of whom were regarded as critically ill, and none were found to have low cortisol levels. In a 1984 study, they reported that mortality fell to 25% in 12 patients treated after etomidate use was discontinued.6
Criticisms of these reports began almost immediately. In The Lancet of June 25, 1983, a letter responding to Ledingham and Watt’s initial report notes “alarm that a technique so useful in critically ill patients can so easily be discredited by a letter so lacking in objective information,” and suggests the effect of a number of additional confounding variables. Another letter suggests that the greater depth of anesthesia obtained with etomidate may explain the mortality. Additional reports by Fellows et al.7 in late 1983 as well as by Chee et al.8 and by Logan and McKee9 in early 1984, all in the British Medical Journal, lent further support to the relation between etomidate and the suppression of adrenal steroidogenesis.
Several recent articles and letters to the editor in various journals question the safety of etomidate for even a single bolus dose in patients with illnesses such as sepsis or trauma that rely upon an adrenal stress response,10–18 citing the risk of causing relative adrenocortical insufficiency after a single bolus. Jackson, in March 2005,12 discusses a number of small randomized studies reporting significant but transient adrenocortical suppression after etomidate administration, and notes that these studies failed to prove that the resultant adrenal dysfunction was insignificant. In an editorial accompanying Jackson’s article,19 the effect of short-term suppression of adrenal synthesis on patient outcomes is described as being unclear, with the authors noting that etomidate is still a useful agent for the induction of unconsciousness, and when combined with muscle relaxation provides the best scenario for rapid, smooth, hemodynamically stable intubation.19 Zed et al.18 conclude that given the significant evidence that etomidate causes transient adrenal insufficiency of uncertain clinical effect, further research is necessary. Bloomfield and Noble11 suggest that a moratorium on the use of etomidate in critically ill patients outside clinical trials may be prudent until its safety is established. In contrast, Crozier20 suggests that “the fervor of the current discussion is excessive, particularly since it is based on rather shaky and perhaps misinterpreted data,” and notes that although the case against etomidate might appear compelling, none of the studies were actually designed to test the effect of etomidate on mortality. Morris and McAllister16 question whether etomidate’s reputation for being safe in emergency anesthesia is justified and ask if it should continue to be used in any practice. Schultz-Stubner,17 in a letter to the editor responding to Vincent and Berre,21 cites recent evidence13 for a single bolus of etomidate in intensive care patients being a major risk factor for the development of relative adrenal insufficiency for at least 24 hours. The author suggests that etomidate should be avoided and replaced by an amnestic dose of a benzodiazepine in combination with an opioid or ketamine to facilitate endotracheal intubation in patients with traumatic brain injury. Berre and Vincent counter with the fact that the decrease in steroidogenesis caused by intravenous administration of a single dose of etomidate has never been demonstrated to be deleterious and that the advantages of etomidate over other agents are numerous.
In summary: Etomidate was determined to be unsafe for long-term use in the ICU shortly after it was introduced in the U.S. in 1982. The question of whether a single bolus dose of etomidate for rapid sequence induction is safe remains a contentious and unresolved issue.
What is the mechanism by which etomidate is thought to affect the adrenal axis?
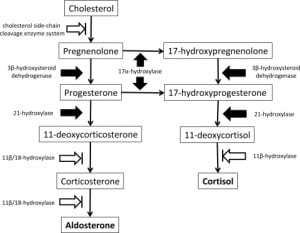
The mechanism of etomidate’s effects on the adrenal axis is through a reversible and concentration-dependent blockade of 11β-hydroxylase and, to a lesser extent, 11β/18-hydroxylase (aldosterone synthase, CYP11B2) and the cholesterol side-chain cleavage enzyme known as cholesterol desmolase, or P450scc. Early characterization of the mechanism was reported in May 1984 by Wagner et al.,22 who described the effect of etomidate infusion on cortisol, and the aldosterone responses to stimulation with adrenocorticotropic hormone (ACTH), in five patients. Wagner et al. also examined the direct effects of etomidate on enzymes in rat cells, noting a marked suppression of adrenal steroidogenesis followed by gradual recovery of glucocorticoid production during four days of observation after stopping etomidate infusion. They concluded by recommending that physicians consider treating selected patients with corticosteroids if etomidate is used for induction. Decreased cortisol and aldosterone levels due to this adrenal suppression have been documented to occur approximately 30 minutes after a single induction dose of etomidate, with the duration of the effect being as long as 24 hours to 48 hours.13,15,23–26
In summary: The mechanism of etomidate’s affect on the adrenal axis is well characterized and uncontroversial.
How has adrenal insufficiency in relation to etomidate use been defined or identified in the literature?
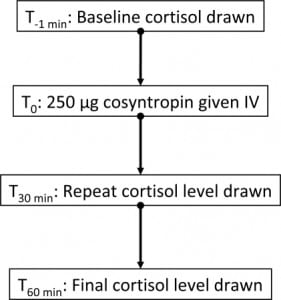
No gold standard exists for the diagnosis of relative adrenal insufficiency. The methods currently used include measuring cortisol concentrations, cosyntropin stimulation testing, and determining various ratios of cortisol, 11-deoxycortisol, ACTH, and other hormone intermediates and derivatives. The cosyntropin test involves measurement of baseline serum cortisol, then the parenteral administration of 250 mcg of synthetic ACTH, followed by serum cortisol measurements at 30 minutes and 60 minutes later. The normal response is considered an increase of greater than 9 mcg of cortisol per deciliter. In an excellent discussion of the assessment of adrenocortical function in the critically ill, Rai et al.27 note that although adrenocortical function is essential for patient survival during critical illness, what constitutes adrenocortical insufficiency in critically ill patients is not clear. Absolute insufficiency, as defined by very low plasma cortisol concentrations, is uncommon in the ICU population (and moreover, it is difficult to determine what constitutes a normal reference range for the critically ill patient). The term “relative adrenocortical insufficiency” (abnormal increases in plasma cortisol concentrations following an ACTH stimulus) is controversial since definitions have been obtained through studies in unstressed volunteers. Critics note that the test is only a measurement of adrenal reserve, not adrenal function, and thus its use to determine adrenal insufficiency in the setting of sepsis is inappropriate. A normal response to the test does not rule out adrenal suppression, because the dose of ACTH used is far higher than normal physiological concentration and may override adrenal resistance to corticotropin, thus producing a false-negative test in patients with mild secondary adrenal insufficiency. An alternative “low-dose” cosyntropin test, using 1 mcg of corticotrophin, has been suggested. A recent consensus statement from the American College of Critical Care Medicine (ACCM) suggests that dysfunction of the hypothalamic-pituitary-adrenal axis in critical illness is best described by the term critical illness–related corticosteroid insufficiency (CIRCI), and that the terms absolute or relative adrenal insufficiency are best avoided in the context of critical illness.28
Regardless of the debate over how best to test for adrenal insufficiency, a clear and unambiguous relation between serum cortisol and mortality in critical illness has not been demonstrated. Plasma cortisol assays tend to vary considerably between different institutions, and circadian rhythms of cortisol levels result in significant fluctuations over a 24-hour period, such that the diagnosis of impaired cortisol secretion may be wrong if based on a single plasma cortisol measurement. Commercial assays only measure the total plasma cortisol, so physiologically significant increases in free cortisol can be missed. For the many reasons noted above, there continues to be uncertainty about the optimal method of detecting clinically significant adrenal insufficiency in acutely ill patients. This is one important factor that makes the relation between etomidate and adverse outcome difficult to address.
In summary: Although it is clear that etomidate inhibits adrenal hormone production, definitions to uniformly characterize levels of adrenal inhibition are still being developed.
What is the evidence that single dose etomidate is associated with subsequent adrenal-cortisol dysfunction?
Multiple studies have evaluated the effect of etomidate on adrenocortical function in patients undergoing elective operative procedures, with the results showing evidence of adrenocortical dysfunction after a single induction dose. In a small observational study of patients undergoing minor surgery, Duthie et al.29 compared the use of etomidate to thiopentone by measuring the effect on synthesis of corticosteroid hormones and ACTH, and found higher levels of plasma 11-deoxycorticosterone (an intermediate molecule in steroid synthesis) at four hours and at 24 hours after administration in patients receiving etomidate. However, the clinical significance of the results remains uncertain in this small study performed in non-septic patients.
In 2001, Schenarts et al.26 performed a prospective randomized controlled (but non-blinded) trial of a small number of consecutive patients presenting to the ED requiring intubation. These patients were randomized to receive a single bolus dose of either midazolam or etomidate during a standardized rapid sequence intubation with succinylcholine. The primary outcome variable was adrenocortical function at 4, 12, and 24 hours post induction, as measured by serum cortisol responses to cosyntropin testing. After exclusion of 13 patients, eight control patients and 10 patients were given etomidate; all controls had a normal response to cosyntropin at four hours, whereas only 30% of the etomidate patients had a normal response. Patients were excluded if steroids were used at any time during the first 24 hours of hospitalization. Although responses to the cosyntropin testing were blunted, baseline cortisol levels, although lower than in controls, tended to be within normal laboratory reference ranges. Normal responses returned at 12 to 24 hours post induction. Although no differences in clinical outcomes were reported, it is interesting to note that for the patients receiving etomidate, the number of hours intubated and the time in ICU were roughly twice the times recorded for the patients receiving midazolam. Also, the hospital length of stay (LOS) was an average two days longer for the etomidate group.
In a retrospective study of patients with septic shock, Mohammad et al.15 examined the incidence of relative adrenal insufficiency after etomidate administration. After identifying 1,207 consecutive patients who had their serum cortisol concentrations measured, these authors were able to include in their study 152 adults with septic shock who underwent cosyntropin stimulation testing; 38 of these patients received etomidate, with an incidence of relative adrenal insufficiency of 76% compared to 51% in the controls (p = 0.0077). Relative adrenal insufficiency was defined as a rise in serum cortisol of less than 9 mcg per deciliter. Patients who did not authorize their medical records to be reviewed for research were excluded; however, this number is not readily available in the published paper. Overall hospital mortality rate was 57%, with etomidate patients having a mortality of 63% and controls having a non-significantly different mortality of 55% (p = 0.45).
In 2008, den Brinker et al.23 reported a retrospective analysis of 60 pediatric patients who presented with meningococcal sepsis between 1997 and 2004. Of these patients, 23 had been intubated with etomidate, eight without etomidate, and 29 had not been intubated. Children receiving etomidate had significantly lower cortisol levels and retained decreased ratios of cortisol to 11-deoxycortisol up to 24 hours after admission. However, severity of illness was not specified in this population. Patients given etomidate also had higher mortality rates (although the clinical outcome is not emphasized in the manuscript, given the limitations in attributing causation through this retrospective study). The authors hypothesize that etomidate “might therefore increase risk of death.”
Vinclair et al.30 performed a prospective observational study of 40 ICU patients without sepsis who received etomidate for endotracheal intubation. The patients were assessed using serial cosyntropin tests and measurements of 11-deoxycortisol levels at 12, 24, 48, and 72 hours after etomidate administration. Adrenal inhibition was defined as a rise of less than 9 mcg per deciliter after ACTH stimulation and an accumulation of 11-deoxycortisol of greater than 8 nmol per liter from baseline. On the basis of this definition, etomidate-related adrenal insufficiency occurred in 80% of the patients at 12 hours, 46% at 24 hours, 9% at 48 hours, and 7% at 72 hours.
In summary: Data from these studies support the contention that a single bolus dose of etomidate results in adrenal-cortisol dysfunction as measured by laboratory analysis.
What is the clinical significance of adrenal insufficiency or dysfunction associated with single-dose etomidate? Where are the data that support or refute the contention that single-dose etomidate is associated with increased mortality or important post-ED clinical outcomes?
The CORTICUS trial reported in 2008 by Sprung et al.31 provided evidence of the clinical significance of adrenal insufficiency associated with single-dose etomidate. This study evaluated the effect of randomized steroid use on the outcomes of septic patients, but did not randomize the use of sedative agents. Of 499 patients randomized to receive either hydrocortisone or placebo, there was no statistically significant difference in mortality (39.2% versus 36.1% in the placebo group) at 28 days for patients who did not respond to corticotropin stimulation testing or for patients who did have a response to corticotropin (28.8% versus 28.7% in the placebo group). When all groups were combined, 86 of 251 patients in the hydrocortisone group versus 78 of 248 patients in the placebo group had died at 28 days (34.3% versus 31.5%). The authors noted more episodes of superinfection, including new sepsis and septic shock, in patients treated with steroids. A post-hoc analysis to evaluate the association between outcomes and the use of etomidate showed that mortality at 28 days increased among patients who received etomidate before randomization (mortality was 45.1% in the hydrocortisone group and 40.0% in the placebo group). Mortality in patients who did not receive etomidate was 31.5% in the hydrocortisone group and 29.6% in the placebo group. This difference was statistically significant, with p = 0.03. In view of the lack of randomization of these patients to receive etomidate or alternative agents, and the consequent limitation in causative attribution, the authors state that “an association between etomidate and the likelihood of adrenal hypo-responsiveness was also found in our study.”
In 2002, Annane et al.32 reported a reduction in mortality after low-dose corticosteroid administration in septic shock patients who failed cosyntropin testing. Almost two years into the study, these investigators amended the eligibility criteria to exclude patients who had received etomidate for endotracheal intubation. Of 72 patients who had received a single dose of etomidate to that point, 68 (94.4%) failed their cosyntropin test, compared to 71% of those who did not receive etomidate. A subgroup analysis of these 68 non-responders revealed significantly higher mortality rates in those who had been randomized to receive placebo versus corticosteroids (75.7% versus 54.8%, respectively; p = .03).
A prospective observational study by Malerba et al.13 also indirectly suggests a statistically significant increase in mortality due to etomidate-associated relative adrenal insufficiency. In this study of 62 patients needing mechanical ventilation for greater than 24 hours, each patient’s cosyntropin response was tested at 24 hours after endotracheal intubation. Multivariate analysis revealed that etomidate was associated with relative adrenal insufficiency (OR 12.21; 95% CI 2.99–49.74) and that the patients with relative adrenal insufficiency demonstrated more organ dysfunction and higher mortality (70.4% versus 31.4%, p < 0.005).
Lipiner-Friedman et al.33 describe data collected from patients in 20 European ICUs. Data for 77% of the patients were extracted from databases of previously published studies, with data for the remaining patients coming from centers participating in (but prior to the actual start of) the CORTICUS study. After evaluation of 562 patients, 477 were retained (due to various exclusionary criteria). Of these patients, 237 received etomidate, resulting in an unadjusted odds ratio for death of 1.53 (95% CI 1.06–2.26), which becomes nonsignificant after adjustment for severity of illness in multivariate analysis.
Ray et al.34 conducted a retrospective review of 159 septic shock patients and assessed the associations between outcome and induction agent, vasopressor use, inotrope use, and steroid use. Vasopressor use, inotrope use, or steroid administration and outcome were found not to be related to the induction agent chosen. The induction agent used and timing of administration did not influence subsequent steroid administration or dose of hydrocortisone. Of 87 patients who started steroid therapy, 58 (67%) died; of 60 patients who received no steroid, 36 (60%) died. Forty-three patients who received etomidate also received steroids; 32 of these patients (74%) died compared with 19 (58%) who died and did not receive steroids (p = 0.121). Hospital mortality was as follows: 69% (etomidate), 56% (propofol), 46% (thiopental), 67% (other), and 81% (none), with no statistically significant differences found. Vasopressor therapy was required less frequently and in smaller doses during endotracheal intubation when etomidate was used to induce anesthesia. The authors concluded that neither clinical outcome nor therapy was affected by the use of etomidate.
In late 2008, Hildreth et al.25 described a prospective and randomized (but nonblinded) study of trauma patients requiring intubation. Patients were randomized to receive either etomidate (0.3 mg per kilogram) or fentanyl (100 mcg) and midazolam (5 mg). Although 61 patients met inclusion criteria, 31 were subsequently excluded from data analysis for various reasons. The average baseline serum cortisol for the etomidate group was 31 mcg per deciliter versus 27 mcg per deciliter for the controls; after medication administration, the serum cortisol average for the etomidate group was 18 versus 28 mcg per deciliter, with ACTH stimulation testing resulting in increases of 4.2 mcg per deciliter for the etomidate group versus 11.2 mcg per deciliter in controls. Hospital LOS for the etomidate group was 13.9 days versus 6.4 for the controls; ICU length of stay was 8.1 days versus three, and ventilator days were 6.3 days versus 1.5 (all comparisons were significant). Mortality between groups was not statistically significant, with 16 of 18 patients in the etomidate group surviving (11% mortality) and all patients in the control group surviving. The large number of excluded patients, combined with the small study size, unfortunately limits the generalizability of these findings.
A retrospective analysis of intubated septic patients found a non-statistically significant increase in mortality in patients given etomidate.35 Of the 46 patients receiving alternative agents or no agent, 18 died, yielding an unadjusted mortality of 39.1% (95% CI 25.5% to 54.6%), while of the 135 receiving etomidate, 63 died, for an unadjusted mortality of 46.7% (95% CI 38.1% to 55.4%).35 A prospective observational study performed by the same investigators followed the outcomes of all patients meeting sepsis criteria intubated over a seven-month period.36 A total of 106 patients with sepsis were intubated over the study period; 74 received etomidate and 32 received alternative agents or no induction agent. In-hospital mortality of patients given etomidate (38%, 95% CI 28% to 49%) was similar to those receiving alternatives (44%, 95% CI 28% to 61%). Surviving patients had a non-statistically significant increase in hospital median LOS after receiving etomidate (10 days) compared to those receiving alternatives (7.5 days), p = 0.08.36 In these studies, however, the retrospective design and lack of randomization of patients limits the possibility of establishing causation.
In 2009, Warner et al.37 performed a retrospective analysis of data previously collected as part of a clinical trial evaluating hypertonic saline (HS) resuscitation in hypotensive blunt trauma patients over the age of 18. The primary endpoint was development of acute respiratory distress syndrome (ARDS). Of the 209 patients initially enrolled in the HS trial, 107 underwent RSI; 13 died within the first 24 hours and could not be assessed for ARDS. There were no statistically significant differences in mortality between those that received etomidate and those that received benzodiazepines (15% versus 25%, p = 0.33). Of the 94 patients that underwent RSI and survived 24 hours, 35 received etomidate and 59 received benzodiazepines at the discretion of either the treating physician or EMS provider. In univariate analyses, the authors found a statistically significant increase in the development of ARDS and Multiple Organ Dysfunction Score (MODS) in patients given etomidate. In multivariable analysis controlling for HS, Acute Physiology and Chronic Health Evaluation (APACHE II) scores, etomidate, massive transfusion, and ISS, the authors found that use of etomidate, an APACHE II score >20, and massive transfusion remained significant predictors of the development of ARDS and MODS. Also, in the most severely injured patients with APACHE II scores of >20, patients that received etomidate had significant increases in hospital LOS, ventilator days, and ICU length of stay. Conclusions regarding etomidate use being causally related to the development of ARDS and MODS were limited because the analysis was post-hoc.
In 2009, Cuthbertson et al.38 reported further details on a sub-group of patients in the CORTICUS trial.31 Their goal was to evaluate the effects of etomidate on corticotropin response and 28-day mortality. Of the 499 patients analyzed, 96 received etomidate within 72 hours of inclusion in the study. Univariate analysis revealed that the number of non-responders to corticotropin was significantly higher in patients who received etomidate than in other patients (61.0%, versus 44.6%, p = 0.004), and mortality was increased in those who received etomidate (OR = 1.70, 95% CI: 1.07–2.68; p = 0.02). The authors also performed two logistic regression analyses. In the first model they adjusted for the treatment group (steroid/placebo), response to corticotropin (responder/non-responder), baseline cortisol value (as continuous variable), and simplified acute physiology score (SAPS II), and found etomidate to have a non-statistically significant effect on mortality (p = 0.06). The second model further added the sequential organ failure assessment (SOFA) score, and revealed a statistically significant increase in mortality in patients who received etomidate (OR 1.75, 95% CI 1.06 to 2.90). In patients receiving etomidate, administration of hydrocortisone did not have a significant effect on mortality. This study remains limited by the non-randomized administration of etomidate, and the limitations inherent in making adjustments for severity of illness.
Jabre et al.39 recently performed a multicenter, controlled, single-blind trial of all non-pregnant patients over the age of 18 who required sedation for emergency rapid sequence intubation. Patients were randomized to receive either 0.3 mg/kg of etomidate or 2 mg/kg of ketamine. The primary outcome was the maximum SOFA score during the first three days in the ICU. In an effort to capture the most critically ill population, patients were excluded from analysis after randomization if they died prior to reaching the hospital or were discharged from the ICU within three days. Of the 655 patients randomized, 181 were excluded due to the aforementioned reasons, four due to missing data, and one because of withdrawn consent, which left 234 patients in the etomidate group and 235 in the ketamine group. The authors found no significant difference between the etomidate and ketamine groups in their maximum SOFA scores during the first three days in the ICU (10.3 versus 9.6, p = 0.056), and in secondary outcomes, no significant difference between the groups in their changes in SOFA scores, 28-day mortality, ventilator-free days, transfusion, fluid, or vasopressor requirements, Glasgow outcome score, or ICU-free days. Ease of intubation was also similar in the two groups, probably as a result of the muscle relaxant effect provided by succinylcholine, which was administered to all intubated patients. The only statistically significant difference between the groups was a decrease in adrenal responsiveness in patients given etomidate. Sub-group analysis of septic patients showed a non-statistically significant 7.2% increased risk of death in patients given etomidate (OR 1.4, 95% CI 0.5 to 3.5). However, the small total number of septic patients (41 receiving etomidate and 35 receiving ketamine) limits the power of this analysis.
In summary: The studies that support the contention that single-dose etomidate is associated with increased mortality or important adverse post-ED clinical outcomes are limited by their observational design. Thus, the strength of any association between etomidate and an adverse outcome can imply, but not prove, causation.
How should etomidate effects in septic patients best be measured?
The optimal method of determining relative adrenal insufficiency in critically ill patients continues to be a matter of debate. Various methods have been suggested, including a randomly drawn cortisol level of less than 15 mcg per deciliter, combined with an increment in cortisol level after a cosyntropin test of less than 9 mcg per deciliter; a cortisol increment after a cosyntropin test of less than 9 mcg per deciliter regardless of basal cortisol levels; and a random cortisol level of less than 25 mcg per deciliter in hypotensive patients or less than 20 mcg per deciliter in normotensive patients.
Rai et al.27 recommend the use of plasma-free cortisol in the assessment of adrenal function in critical illness and further suggest that the low-dose corticotropin test is more sensitive than the conventional high-dose test. Cortisol is bound to an alpha-globulin called transcortin, or corticosteroid-binding globulin, as well as albumin, and also exists in a free form. The free hormone is the active form, and less than 5% exists as free cortisol in the plasma at normal levels of total plasma cortisol. During critical illness, levels of cortisol-binding globulin decrease, and free cortisol levels may increase secondary to the cleavage of cortisol-binding globulin by neutrophil elastase. However, because commercial assays only measure the total plasma cortisol, a physiologically significant increase in free cortisol can be missed. Serum total cortisol levels can be reduced in hypoproteinemic patients, while serum-free cortisol levels are elevated. Rai et al.27 suggest that baseline free cortisol levels of 2 mcg per deciliter should be considered the threshold level that identifies patients at risk for adrenal insufficiency during critical illness, and that a corticotropin stimulated serum-free cortisol concentration of 3.1 mcg per deciliter or greater defines a normal response in critically ill patients.
According to a recently published ACCM consensus statement,28 CIRCI is best diagnosed (after administration of 250 mcg of cosyntropin) by a delta cortisol of <9 mcg/dL or a random total cortisol of <10 mcg/dL. Measurement of free cortisol is not recommended for routine use, because although the free cortisol assay has some advantage over the total serum cortisol, this test is not readily available. Furthermore, the normal range of free cortisol in critically ill patients is currently unclear.28 With regard to the use of supplemental steroids, the consensus is that clinical criteria, as opposed to ACTH stimulation testing, should be used to identify which patients with septic shock or ARDS should receive glucocorticoids.28
In summary: Although the clinical significance of adrenocortical suppression caused by etomidate remains uncertain, measurments of delta cortisol after administration of 250 mcg of cosyntropin, or measurement of a random cortisol level, appear reasonable to identify critical illness related corticosteroid insufficiency (CIRCI).
What are alternative induction agents? What are the advantages and disadvantages of these agents relative to etomidate?
Both ketamine and benzodiazepines, particularly midazolam, appear to be suitable alternatives to etomidate for most cases of rapid sequence intubation of septic patients. The recommended dose of midazolam for induction is between 0.1 and 0.3 mg/kg (although the package insert notes initial dose requirements of up to 0.35 mg/kg with resistant cases requiring up to 0.6 mg/kg). The recommended dosage for diazepam is 0.2–0.5 mg/kg, methohexital 1.0–3.0 mg/kg, and thiopental 3.0–5.0 mg/kg. As noted by Sagarin et al.,40 in their review of the National Emergency Airway Registry database, under-dosing with midazolam is common, despite its minimal hypotensive effects, even at high doses used on patients with limited cardiac reserve.
The possibility has been raised that alternative agents, such as midazolam, may have increased hypotensive effects compared to etomidate.41 However, the literature that suggests such adverse outcomes is itself limited by observational analyses that suggest associations but cannot prove causality.42–47 Despite any adjustments made to account for severity of illness, the fact remains that unmeasured confounders in these studies are likely to have influenced outcome.
Ketamine has been suggested to have an excellent hemodynamic profile, making it a reasonable alternative to etomidate, at least in some patients.41 Evidence supporting the safety of ketamine for rapid sequence intubation is now available in the form of a multicenter randomized controlled trial (discussed earlier), which concluded that ketamine is a safe and valuable alternative to etomidate.39Nevertheless, prior evidence suggesting adverse cardiovascular effects from ketamine may leave some physicians hesitant to adopt it as a substitute for etomidate in all patients, particularly in patients with known or suspected cardiovascular disease.48,49,50,51 Additionally, ketamine has historically been avoided by clinicians following actual or potential brain injury due to its potential to elevate intracranial pressure, despite recent reviews questioning the significance of this effect on outcome.52
In summary: Both midazolam and ketamine appear to be suitable alternatives to etomidate. Given the limitations in available evidence, a strong recommendation for any particular agent at this time is not possible; however, with the need to balance theoretical harms and benefits in the presence of data supporting the non-inferiority of alternative agents which do not have similar theoretical risks associated with them, we suggest that further studies to support continued widespread use of etomidate in sepsis are warranted. As with any therapeutic decision, practitioners must choose between agents. There is sufficient reason to think that ketamine and midazolam are safe alternatives and that in the context of clear sepsis or septic shock, these agents should be considered. Practitioners would be well served by becoming familiar with the use of more than one agent while awaiting further definitive data.
What future work is needed to further clarify the characteristics of etomidate as it is currently used in patients with sepsis?
It is possible, or even likely, that the largest randomized study to date of etomidate was underpowered for detection of mortality differences in septic patients, and that further study is warranted.39 Some doubt remains over the safety of using etomidate as an induction agent for rapid sequence intubation in septic patients.10–12,14–20,23,35,53–57 If etomidate does in fact have a negative influence on patient outcome, this will only be proved by conducting randomized, controlled studies with sufficient power to detect what may be small but clinically important differences in outcomes. Such studies will undoubtedly require large, multicenter trials. A recent manuscript describes the creation of a prioritized Emergency Medical Services for Children research agenda specific for multicenter research, and concludes that the creation of the Pediatric Emergency Care Applied Research Network (PECARN) provides a means to answer important clinical controversies, mainly because it will facilitate the procedures necessary for conducting large-scale randomized, controlled trials and observational studies.58 Likewise, the formation of the Resuscitation Outcomes Consortium now provides for a well-developed infrastructure for the conduct of multicenter trials, and offers great promise for the resuscitation community.59
Because the patient population requiring emergent intubation precludes their informed consent being obtained prior to treatment, studies of etomidate in these patients require exception from informed consent. Historically, this has been a challenging proposition, but recent recommendations for implementation of community consultation and public disclosure under the Food and Drug Administration’s “Exception From Informed Consent Requirements For Emergency Research” now provides guidance that will probably increase the ability of investigators to obtain IRB approval to pursue research on etomidate.60 A study in the United States (ClinicalTrials.gov Identifier NCT00441792) is in its final stages, and the results of this study may shed further light on the effects of etomidate.
CONCLUSION
The observational nature of almost all available data suggesting adverse outcomes from etomidate does not currently support abandoning its use for rapid sequence induction. On the other hand, given the limitations in the available evidence, strong support for any particular agent at this point cannot reasonably be made. The only published randomized, controlled trial evaluating etomidate (comparing it to ketamine) did not show a statistically significant difference in outcomes. However, the possibility exists that this trial was underpowered for the subgroup of patients with sepsis. Because etomidate decreases the cortisol response, and because cortisol production in some settings may be clinically important, practitioners should be familiar with the available evidence while awaiting newer studies that may clarify these issues. The authors do not currently have unanimous agreement on a single best agent but feel that the potential for adverse effects of etomidate in clearly septic patients without cardiovascular disease warrants consideration of ketamine or midazolam in these patients.
Footnotes
Supervising Section Editor: Jeffrey Sankoff, MD
Submission history: Submitted April 9, 2009; Revision Received November 3, 2009; Accepted November 29, 2009
Full text available through open access at http://escholarship.org/uc/uciem_westjem
Address for Correspondence: Erik Kulstad, MD, MS, Advocate Christ Medical Center, Department of Emergency Medicine, 4440 W. 95th St., Oak Lawn, IL 60453
Email: kulstad@uic.edu
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
1. Ledingham IM, Finlay WEI, Watt I, et al. Etomidate and adrenocortical function. Lancet.1983;321:1434.
2. Ledingham IM, Watt I. Influence of sedation on mortality in critically ill multiple trauma patients.Lancet. 1983;321:1270. [PubMed]
3. Preziosi P, Vacca M. Etomidate and corticotrophic axis. Archives internationales de pharmacodynamie et de therapie. 1982;256:308–310. [PubMed]
4. Finlay WE, McKee JI. Serum cortisol levels in severely stressed patients. Lancet. 1982;1:1414–5.[PubMed]
5. McKee JI, Finlay WE. Cortisol replacement in severely stressed patients. Lancet. 1983;321:484.[PubMed]
6. Watt I, Ledingham IM. Mortality amongst multiple trauma patients admitted to an intensive therapy unit. Anaesthesia. 1984;39:973–81. [PubMed]
7. Fellows IW, Bastow MD, Byrne AJ, et al. Adrenocortical suppression in multiply injured patients: a complication of etomidate treatment. Br Med J (Clin Res Ed) 1983;287:1835–7.
8. Chee HD, Bronsveld W, Lips PTAM, et al. Adrenocortical suppression in multiply injured patients: a complication of etomidate treatment – Reply. Br Med J (Clin Res Ed) 1984;288:485.
9. Logan RW, McKee JI. Adrenocortical suppression in multiply injured patients: a complication of etomidate treatment – Reply. Br Med J (Clin Res Ed) 1984;288:485–486.
10. Bloomfield R, Noble DW. Etomidate and fatal outcome–even a single bolus dose may be detrimental for some patients. Br J Anaesth. 2006;97:116–7. [PubMed]
11. Bloomfield R, Noble DW. Etomidate, pharmacological adrenalectomy and the critically ill: a matter of vital importance. Crit Care. 2006;10:161. [PMC free article] [PubMed]
12. Jackson WL., Jr Should we use etomidate as an induction agent for endotracheal intubation in patients with septic shock?: a critical appraisal. Chest. 2005;127:1031–8. [PubMed]
13. Malerba G, Romano-Girard F, Cravoisy A, et al. Risk factors of relative adrenocortical deficiency in intensive care patients needing mechanical ventilation. Intensive Care Med. 2005 Mar;31:388–92.[PubMed]
14. McConachie I. Etomidate controversies in emergency medicine. Ann Emerg Med. 2007;50:200–201. [PubMed]
15. Mohammad Z, Afessa B, Finkielman JD. The incidence of relative adrenal insufficiency in patients with septic shock after the administration of etomidate. Crit Care. 2006;10:R105. [PMC free article][PubMed]
16. Morris C, McAllister C. Etomidate for emergency anaesthesia; mad, bad and dangerous to know?Anaesthesia. 2005;60:737–40. [PubMed]
17. Schulz-Stubner S. Sedation in traumatic brain injury: avoid etomidate Crit Care Med 2005.332723; author reply 2723. [PubMed]
18. Zed P, Mabasa V, Slavik R, et al. Etomidate for rapid sequence intubation in the emergency department: Is adrenal suppression a concern? Can J Emerg Med. 2006;8:347–50.
19. Murray H, Marik PE. Etomidate for endotracheal intubation in sepsis: acknowledging the good while accepting the bad. Chest. 2005;127:707–9. [PubMed]
20. Crozier TA. Fatal flaws in the case against etomidate for induction. Br J Anaesth. 2006;97
21. Vincent JL, Berre J. Primer on medical management of severe brain injury. Crit Care Med.2005;33:1392–99. [PubMed]
22. Wagner RL, White PF, Kan PB, et al. Inhibition of adrenal steroidogenesis by the anesthetic etomidate. N Engl J Med. 1984;310:1415–21. [PubMed]
23. den Brinker M, Hokken-Koelega AC, Hazelzet JA, et al. One single dose of etomidate negatively influences adrenocortical performance for at least 24h in children with meningococcal sepsis.Intensive Care Med. 2008;34:163–8. [PMC free article] [PubMed]
24. den Brinker M, Joosten KF, Liem O, et al. Adrenal insufficiency in meningococcal sepsis: bioavailable cortisol levels and impact of interleukin-6 levels and intubation with etomidate on adrenal function and mortality. J Clin Endocrinol Metab. 2005;90:5110–7. [PubMed]
25. Hildreth AN, Mejia VA, Maxwell RA, et al. Adrenal suppression following a single dose of etomidate for rapid sequence induction: a prospective randomized study. The Journal of trauma.2008;65:573–9. [PubMed]
26. Schenarts CL, Burton JH, Riker RR. Adrenocortical dysfunction following etomidate induction in emergency department patients. Acad Emerg Med. 2001;8:1–7. [PubMed]
27. Rai R, Cohen J, Venkatesh B. Assessment of adrenocortical function in the critically ill. Crit Care Resusc. 2004;6:123–9. [PubMed]
28. Marik PE, Pastores SM, Annane D, et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med.2008;36:1937–49. [PubMed]
29. Duthie DJ, Fraser R, Nimmo WS. Effect of induction of anaesthesia with etomidate on corticosteroid synthesis in man. Br J Anaesth. 1985;57:156–9. [PubMed]
30. Vinclair M, Broux C, Faure P, et al. Duration of adrenal inhibition following a single dose of etomidate in critically ill patients. Intensive Care Med. 2008;34:714–9. [PubMed]
31. Sprung CL, Annane D, Keh D, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358:111–24. [PubMed]
32. Annane D, Sebille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862–71. [PubMed]
33. Lipiner-Friedman D, Sprung CL, Laterre PF, et al. Adrenal function in sepsis: the retrospective Corticus cohort study. Crit Care Med. 2007;35:1012–8. [PubMed]
34. Ray DC, McKeown DW. Effect of induction agent on vasopressor and steroid use, and outcome in patients with septic shock. Crit Care. 2007;11:R56. [PMC free article] [PubMed]
35. Tekwani KL, Watts HF, Chan CW, et al. The effect of single-bolus etomidate on septic patient mortality: a retrospective review. West J Emerg Med. 2008;9:195–200. [PMC free article] [PubMed]
36. Tekwani KL, Watts HF, Rzechula KH, et al. A prospective observational study of the effect of etomidate on septic patient mortality and length of stay. Acad Emerg Med. 2009;16:11–14.[PubMed]
37. Warner KJ, Cuschieri J, Jurkovich GJ, et al. Single-dose etomidate for rapid sequence intubation may impact outcome after severe injury. J Trauma. 2009;67:45–50. [PubMed]
38. Cuthbertson BH, Sprung CL, Annane D, et al. The effects of etomidate on adrenal responsiveness and mortality in patients with septic shock. Intensive Care Med. 2009
39. Jabre P, Combes X, Lapostolle F, et al. Etomidate versus ketamine for rapid sequence intubation in acutely ill patients: a multicentre randomised controlled trial. Lancet. 2009;374:293–300.[PubMed]
40. Sagarin MJ, Barton ED, Sakles JC, et al. Underdosing of midazolam in emergency endotracheal intubation. Acad Emerg Med. 2003;10:329–38. [PubMed]
41. Walls RM, Osborn TM. Special Report: Should Etomidate Be Used as an Induction Agent for Patients with Sepsis? Journal Watch Emergency Medicine http://emergency-medicine.jwatch.org/cgi/content/full/2008/201/5 Accessed: August 12, 2009.
42. Jones AE, Yiannibas V, Johnson C, et al. Emergency department hypotension predicts sudden unexpected in-hospital mortality: a prospective cohort study. Chest. 2006;130:941–6. [PubMed]
43. Mort TC. Complications of emergency tracheal intubation: hemodynamic alterations–part I. J Intensive Care Med. 2007;22:157–65. [PubMed]
44. Franklin C, Samuel J, Hu TC. Life-threatening hypotension associated with emergency intubation and the initiation of mechanical ventilation. Am J Emerg Med. 1994 Jul;12:425–8. [PubMed]
45. Schwartz DE, Matthay MA, Cohen NH. Death and other complications of emergency airway management in critically ill adults. A prospective investigation of 297 tracheal intubations.Anesthesiology. 1995;82:367–76. [PubMed]
46. Choi YF, Wong TW, Lau CC. Midazolam is more likely to cause hypotension than etomidate in emergency department rapid sequence intubation. Emerg Med J. 2004;21:700–2. [PMC free article][PubMed]
47. Davis DP, Kimbro TA, Vilke GM. The use of midazolam for prehospital rapid-sequence intubation may be associated with a dose-related increase in hypotension. Prehosp Emerg Care. 2001;5:163–8.[PubMed]
48. Christ G, Mundigler G, Merhaut C, et al. Adverse cardiovascular effects of ketamine infusion in patients with catecholamine-dependent heart failure. Anaesthesia and intensive care. 1997;25:255–9. [PubMed]
49. Pagel PS, Kampine JP, Schmeling WT, et al. Ketamine depresses myocardial contractility as evaluated by the preload recruitable stroke work relationship in chronically instrumented dogs with autonomic nervous system blockade. Anesthesiology. 1992;76:564–72. [PubMed]
50. Lippmann M, Appel PL, Mok MS, et al. Sequential cardiorespiratory patterns of anesthetic induction with ketamine in critically ill patients. Crit Care Med. 1983;11:730–4. [PubMed]
51. Lippmann M, Kakazu C. Intubating ICU patients with ketamine: adverse effects that can occur.Chest. 2007;132:2054. [PubMed]
52. Morris C, Perris A, Klein J, et al. Anaesthesia in haemodynamically compromised emergency patients: does ketamine represent the best choice of induction agent? Anaesthesia. 2009;64:532–9.[PubMed]
53. Cotton BA, Guillamondegui OD, Fleming SB, et al. Increased risk of adrenal insufficiency following etomidate exposure in critically injured patients Arch Surg 2008. 14362–67.67; discussion 67.[PubMed]
54. Mullins ME, Theodoro DL. Lack of evidence for adrenal insufficiency after single-dose etomidateArch Surg 2008. 143808–9.9; author reply 809. [PubMed]
55. Oglesby AJ. Should etomidate be the induction agent of choice for rapid sequence intubation in the emergency department? Emerg Med J. 2004;21:655–9. [PMC free article] [PubMed]
56. Sacchetti A. Etomidate: not worth the risk in septic patients. Ann Emerg Med. 2008;52:14–6.[PubMed]
57. Walls RM, Murphy MF. Clinical controversies: etomidate as an induction agent for endotracheal intubation in patients with sepsis: continue to use etomidate for intubation of patients with septic shock. Ann Emerg Med. 2008;52:13–4. [PubMed]
58. Miller SZ, Rincon H, Kuppermann N. Revisiting the emergency medicine services for children research agenda: priorities for multicenter research in pediatric emergency care. Acad Emerg Med.2008;15:377–83. [PubMed]
59. Morley P. Steady as a ROC: the Resuscitation Outcomes Consortium. Resuscitation.2008;78:105–6. [PubMed]
60. Halperin H, Paradis N, Mosesso V, Jr, et al. Recommendations for implementation of community consultation and public disclosure under the Food and Drug Administration’s «Exception from informed consent requirements for emergency research»: a special report from the American Heart Association Emergency Cardiovascular Care Committee and Council on Cardiopulmonary, Perioperative and Critical Care. Endorsed by the American College of Emergency Physicians and the Society for Academic Emergency Medicine. Circulation. 2007 Oct 16;116:1855–63. [PubMed]


